Label: WANDER PACK UP AND GLOW- titanium dioxide, zinc oxide lotion
- NDC Code(s): 82510-000-01, 82510-000-02
- Packager: Wander Beauty Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
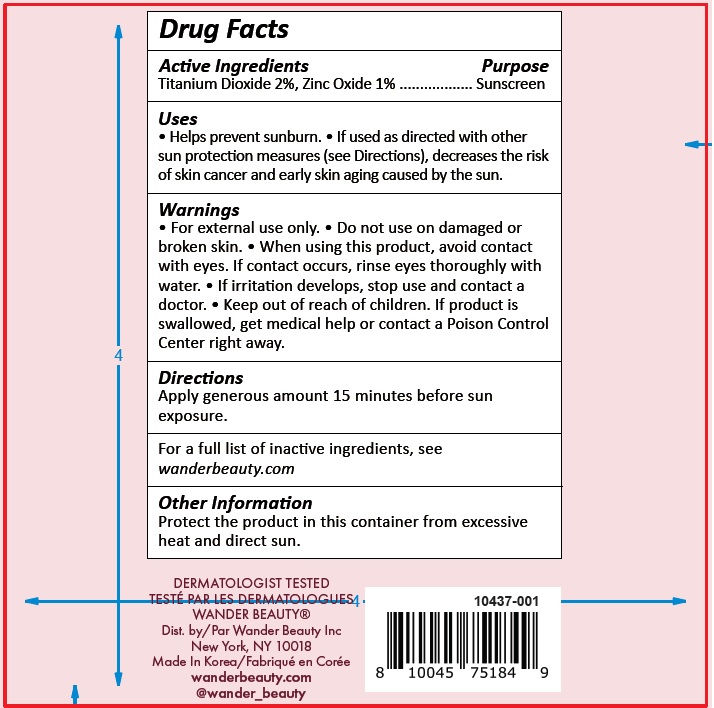
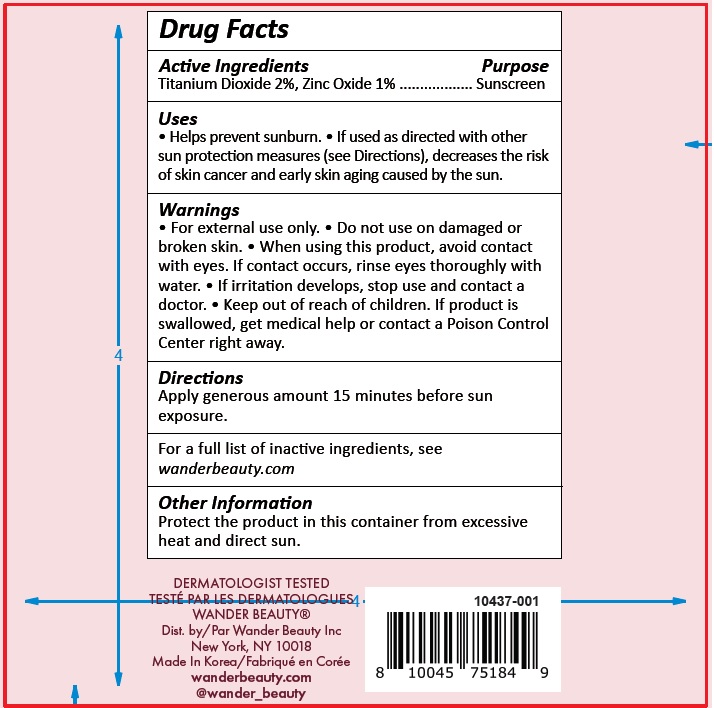
- Drug Facts
- Active Ingredients
- Uses
- Warnings
- Directions
-
Inactive Ingredients
Water/Aqua/Eau, Butyloctyl Salicylate, Octyldodecyl Neopentanoate, Ethylhexyl Isononanoate, Glycerin, Polyester-7, Polymethylsilsesquioxane, Neopentyl Glycol Diheptanoate, Cetyl Alcohol, Glyceryl Stearate, Squalane, Cholecalciferol (Vitamin D3), PEG-75 Stearate, Phenoxyethanol, Hydrolyzed Sodium Hyaluronate, Pyrus Malus (Apple) Fruit Extract, Bisabolol, C12-15 Alkyl Benzoate, Dimethiconol/ Caprylylsilsesquioxane /Silicate Crosspolymer, Caprylyl Glycol, Allantoin, Titanium Dioxide (CI 77891), Iron Oxides (CI 77492), Triceteareth-4 Phosphate, Lysolecithin, Sclerotium Gum, Ceteth-20, Steareth-20, Synthetic Fluorphlogopite, Iron Oxides (CI 77491), Sodium Hyaluronate, Trilaureth-4 Phosphate,
Ethylhexylglycerin, Hexylene Glycol, Disodium EDTA, Dimethiconol/Propylsilsesquioxane/Silicate Crosspolymer, Xanthan Gum, Pullulan, Mica, Avena Sativa (Oat) Kernel Extract, Silica, Sodium Starch Octenylsuccinate, Maltodextrin, Sodium Citrate, Tin Oxide, Potassium Sorbate, Camellia Sinensis Leaf Extract, Lecithin, Zea Mays (Corn) Oil, Sucrose, Dicrateria Rotunda Oil, Ruttnera Lamellosa Oil, Sodium Ascorbate, Tocopherol - Other Information
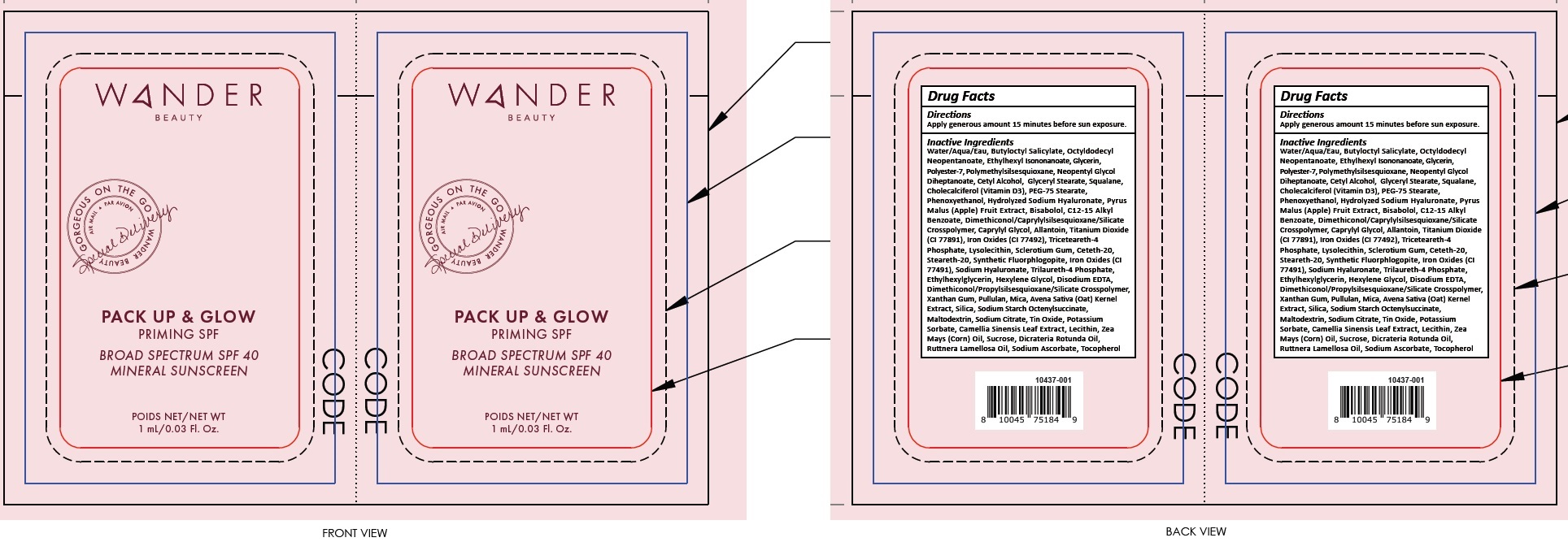
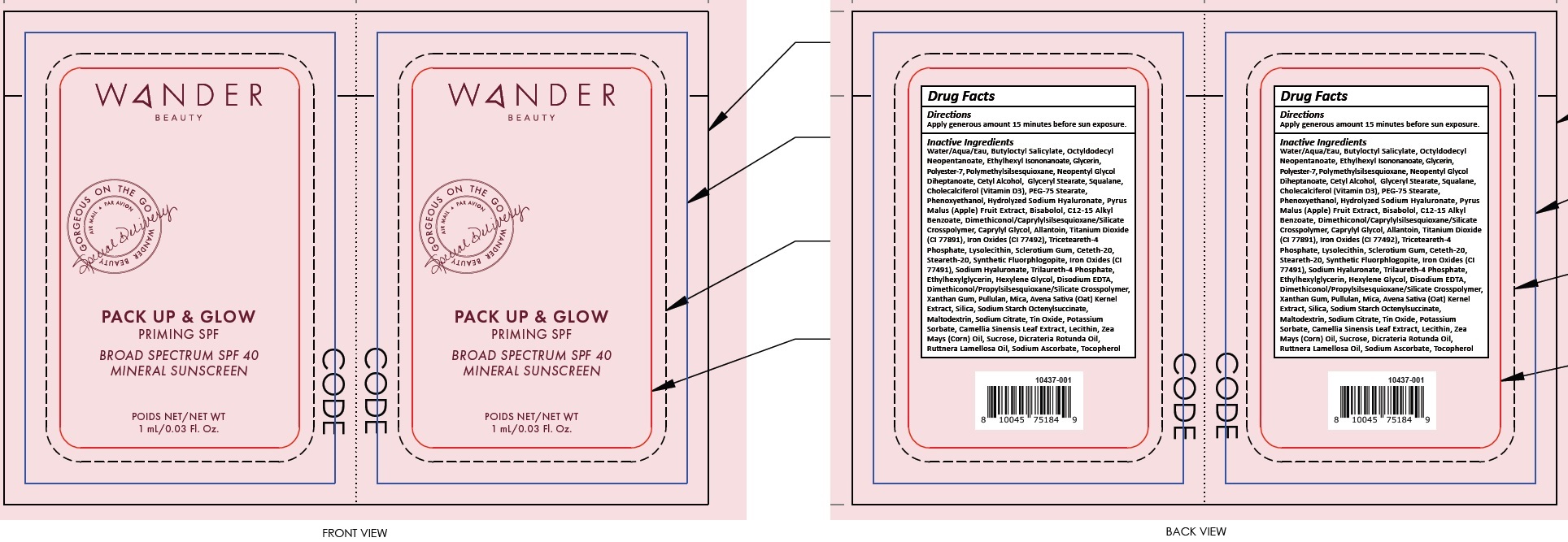
- Package Labeling:82510-000-02
- Package Labeling:82510-000-01
-
INGREDIENTS AND APPEARANCE
WANDER PACK UP AND GLOW
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82510-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 20 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) ETHYLHEXYL ISONONANOATE (UNII: I6KB4GE3K4) GLYCERIN (UNII: PDC6A3C0OX) POLYESTER-7 (UNII: 0841698D2F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SQUALANE (UNII: GW89575KF9) CHOLECALCIFEROL (UNII: 1C6V77QF41) PEG-75 STEARATE (UNII: OT38R0N74H) PHENOXYETHANOL (UNII: HIE492ZZ3T) APPLE (UNII: B423VGH5S9) LEVOMENOL (UNII: 24WE03BX2T) BENZOIC ACID (UNII: 8SKN0B0MIM) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE RED (UNII: 1K09F3G675) TRICETEARETH-4 PHOSPHATE (UNII: 69534Y66NO) BETASIZOFIRAN (UNII: 2X51AD1X3T) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TRILAURETH-4 PHOSPHATE (UNII: M96W2OLL2V) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) XANTHAN GUM (UNII: TTV12P4NEE) PULLULAN (UNII: 8ZQ0AYU1TT) MICA (UNII: V8A1AW0880) OAT (UNII: Z6J799EAJK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MALTODEXTRIN (UNII: 7CVR7L4A2D) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) STANNIC OXIDE (UNII: KM7N50LOS6) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CORN OIL (UNII: 8470G57WFM) SUCROSE (UNII: C151H8M554) DICRATERIA ROTUNDA OIL (UNII: Z0DWU9R4GM) RUTTNERA LAMELLOSA OIL (UNII: 5XZ38R4SUT) SODIUM ASCORBATE (UNII: S033EH8359) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82510-000-01 1 in 1 BLISTER PACK 04/01/2022 1 1 mL in 1 PACKET; Type 0: Not a Combination Product 2 NDC:82510-000-02 1 in 1 BOX 04/01/2022 2 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2022 Labeler - Wander Beauty Inc. (044376185)