Label: LORATADINE capsule, liquid filled
- NDC Code(s): 69452-211-03, 69452-211-07, 69452-211-16, 69452-211-26
- Packager: Bionpharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 8, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
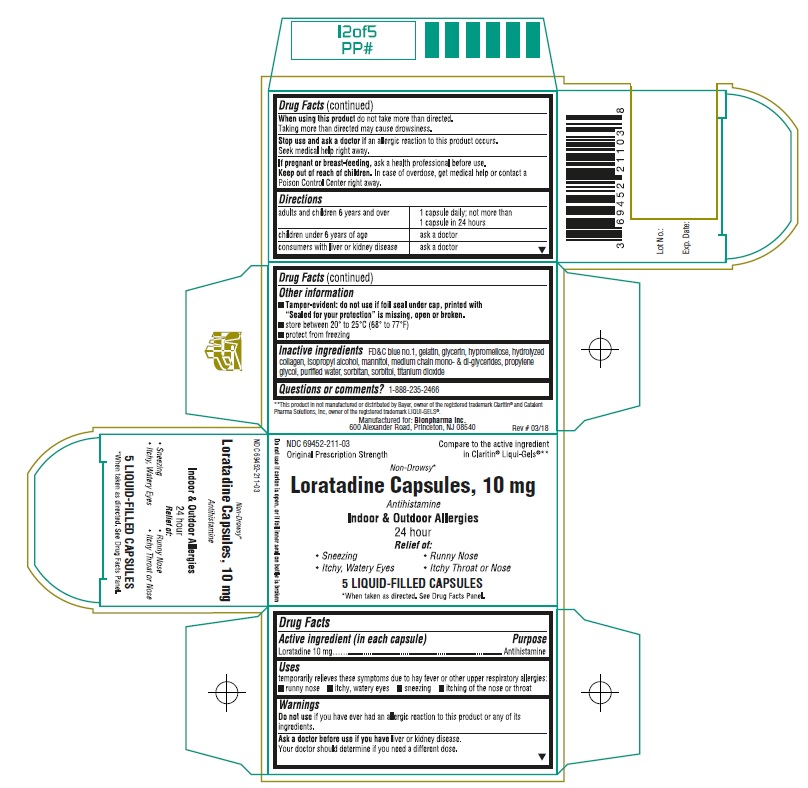
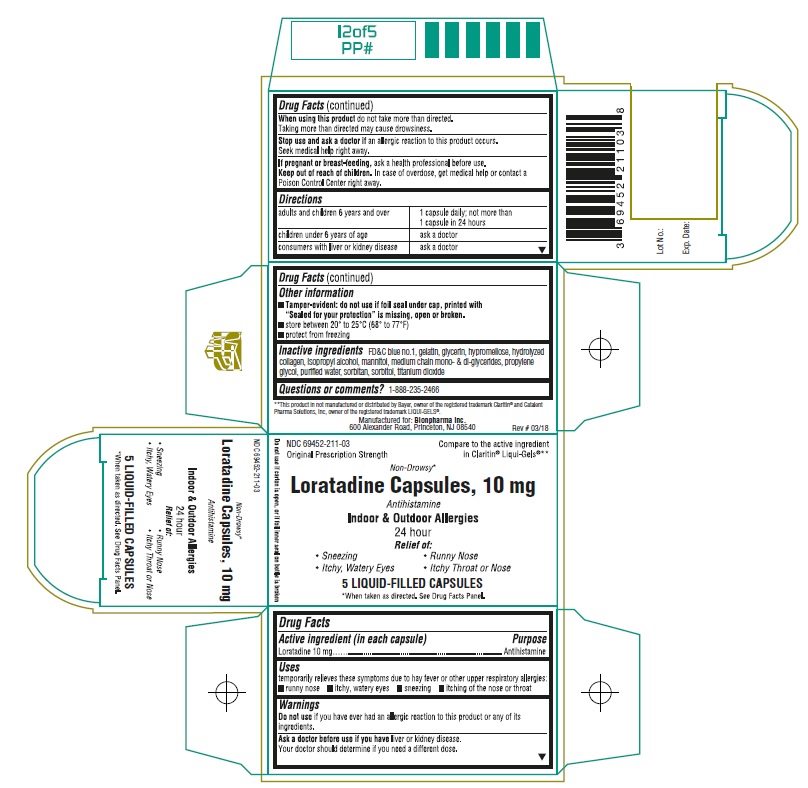
- Active ingredient (in each capsule)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
-
Other information
- Tamper-evident: do not use if foil seal under cap, printed with “Sealed for your protection” is missing, open or broken. (For Bottle Labels and Cartons)
- Safety sealed: do not use if individual blister unit printed with Loratadine Capsule, 10 mg is open or torn. (For Blister Carton)
- store between 20° to 25°C (68° to 77°F)
- protect from freezing
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
NDC 69452-211-03
Compare to the active ingredient in Claritin® Liqui-Gels®**
Original Prescription Strenght
Non-Drowsy*
Loratadine Capsules, 10 mg
Antihistamine
Indoor & Outdoor Allergies
24 hour
Relief of:
• Sneezing
• Runny Nose
• Itchy, Watery Eyes
• Itchy Throat or Nose
5 LIQUID-FILLED CAPSULES
*When taken as directed. See Drug Facts Panel
Do not use if carton is open, or if foil inner seal on bottle is broken
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69452-211 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSES (UNII: 3NXW29V3WO) HYDROLYSED BOVINE COLLAGEN (ENZYMATIC; 2000-5000 MW) (UNII: 5WE8P977RQ) ISOPROPYL ALCOHOL (UNII: ND2M416302) MANNITOL (UNII: 3OWL53L36A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITAN (UNII: 6O92ICV9RU) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue Score no score Shape OVAL Size 10mm Flavor Imprint Code 446 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69452-211-03 1 in 1 CARTON 03/01/2019 1 5 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69452-211-16 1 in 1 CARTON 03/01/2019 2 50 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:69452-211-26 1 in 1 CARTON 03/01/2019 3 200 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:69452-211-07 1 in 1 CARTON 03/01/2019 4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202538 03/01/2019 Labeler - Bionpharma Inc. (079637826) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations Patheon Softgels Inc. 002193829 manufacture(69452-211)