Label: REVEOS SELECT CPD/AS-5 RED CELL PRESERVATIVE FOR COLLECTION OF BLOOD- dextrose monohydrate, trisodium citrate dihydrate, anhydrous citric acid, and sodium phosphate, monobasic, unspecified form solution
- NDC Code(s): 82906-506-01, 82906-506-02, 82906-506-16
- Packager: Terumo BCT Vietnam CO., Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
REVEOS® SELECT SET
CPD WITH AS-5 RED CELL PRESERVATIVE SOLUTION FOR COLLECTION OF 500 mL OF BLOOD
Terumo BCT, Inc.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use the Reveos SELECT set safely and effectively. See full prescribing information for the Reveos SELECT set.
REVEOS SELECT SET
Sterile Fluid
PVC Plasticized With DEHP Bag
Initial U.S. Approval: TBDINDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Do not reuse. (5)
- Inspect the packaging and the blood bag set prior to use. (5)
- The blood bag set is no longer sterile under certain conditions. (5)
- Do not process whole blood less than 2 hours after collection. (5)
- Residual leukocytes are not intended for transfusion. (5)
- Use aseptic technique during blood collection to ensure donor safety and product quality. (5)
- Do not vent the blood bag set. (5)
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Terumo BCT, Inc. at 1-877-339-4228 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
USE IN SPECIFIC POPULATIONS
The Reveos SELECT set has not been studied in controlled clinical trials with specific populations. (8)
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
6. ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
16 HOW SUPPLIED/STORAGE AND HANDLING
STORAGE
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Set Diagram
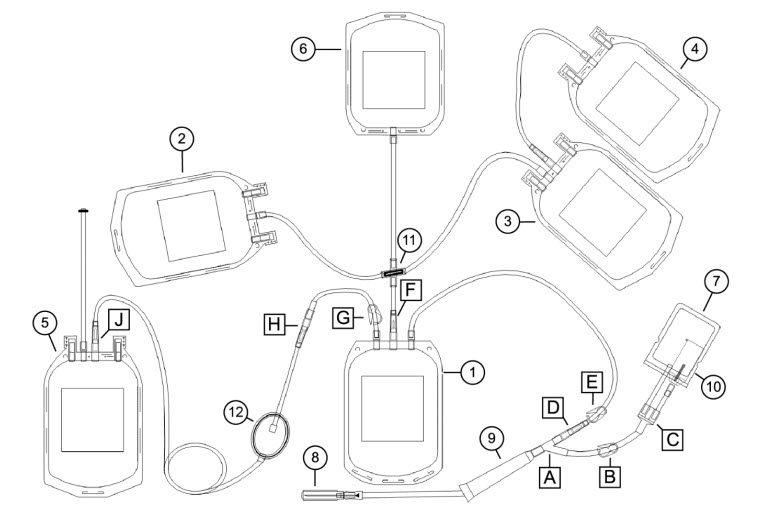
1 Whole blood bag 7 Sample/diversion bag A, C: Y connector 2 Interim platelet unit (IPU) bag 8 Needle B, G: Blue clamp 3 Plasma bag 9 Needle injury protector (NIP) D, F, H, J: CLIKTIP frangible connector 4 Plasma cryoprecipitate reduced bag 10 Sample tube holder/luer adapter E: White clamp 5 Red blood cell (RBC) bag 11 Cross-connector 6 Residual leukocyte bag 12 RBC leukoreduction filter This document provides blood collection and post-processing instructions specific to the Reveos SELECT set. For warnings, cautions, and instructions on processing whole blood with the Reveos system, see the Reveos system operator's manual.
Note: Refer to the instructions for use provided by the manufacturer of your tubing sealer to ensure that the tubing sealer is appropriate for the tubing on the blood bag set.
Note: You must validate the process for producing, storing, and handling Cryoprecipitated Antihemophilic Factor within your institution's standard operating procedure (SOP), including procedures for using the plasma cryoprecipitate reduced bag.
Blood Collection
Required Supplies:
- •
- Scale and/or blood mixing device
- •
- Tubing sealer
- •
- Evacuated blood collection tubes
- 1.
- If it is part of your institution's SOP, make a loose knot in the collection tubing between white clamp E and the whole blood bag.
- 2.
- Separate the sample/diversion bag from the other bags.
- 3.
- Load the bags onto a scale and/or blood mixing device according to your institution's SOP. Ensure that the whole blood bag and the sample bag are lower than the donor's arm.
- 4.
- Close blue clamp B.
- 5.
- Apply a blood pressure cuff or a tourniquet to the donor's arm.
- 6.
- Prepare the venipuncture site.
- 7.
- Twist the needle cap until the resistance stops and then pull the needle cap straight off.
- 8.
- Perform the venipuncture according to your institution's SOP.
- 9.
- Open blue clamp B.
- 10.
- Position the sample/diversion bag with blue clamp B at the top and the sample tube holder/luer adapter at the bottom (see Figure 1). Allow the desired volume of whole blood to flow into the sample/diversion bag. The nominal volume of the sample bag is 60 mL. When the volume reaches the marking, approximately 40 mL has been collected.
- 11.
- Close blue clamp B.
- 12.
- Break CLIKTIP D to allow the whole blood to flow into the whole blood bag. Bend the CLIKTIP in both directions to ensure that you break it completely.
- 13.
- Seal the sample/diversion tubing as near as possible to Y connector A. Seal the tubing according to your institution's SOP.
- 14.
- Invert the sample/diversion bag and transfer donor blood samples from the sample/diversion bag, using evacuated blood collection tubes. Transfer samples as soon as possible after venipuncture to avoid possible clot formation in the sample/diversion bag.
- a.
- Hold the sample/diversion bag and sample tube holder/luer adapter in one hand with blue clamp B positioned at the bottom (see Figure 2).
- b.
- Using the other hand, insert a blood collection tube firmly into the sample tube holder/luer adapter. After filling the blood collection tube, remove the tube from the sample tube holder. Repeat the process to take additional samples.
- 15.
- Mix the whole blood and the anticoagulant during collection according to your institution's SOP.
- 16.
- Collect the target volume of blood ± 10%, as specified on the whole blood bag label.
- 17.
- Close white clamp E and perform one of the following steps:
- If you made a knot in the collection tubing in step 1, tighten the knot firmly.
- If you did not make a knot in step 1, seal the collection tubing near CLIKTIP D.
- 18.
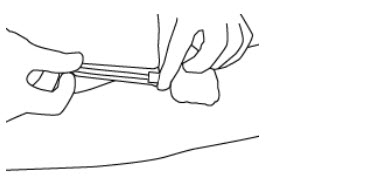
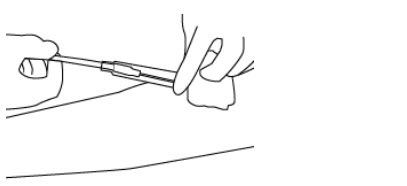
- Hold the needle hub and then slide the NIP partially over the needle hub (see Figure 3).
- 19.
- Gently pull on the tubing to withdraw the needle from the donor's arm and into the NIP until the needle locks securely (see Figure 4).
- 20.
- Seal the collection tubing near CLIKTIP D if you did not do so in step 17. Disconnect the sealed tubing that includes the needle and the sampling assembly. Dispose of the needle, the NIP, and the sample tube holder safely according to your institution's SOP and/or local regulations.
- 21.
- Immediately after collection is complete, invert the whole blood bag several times to thoroughly mix the whole blood and the anticoagulant.
- 22.
- If required, strip the blood in the collection tubing into the whole blood bag according to your institution's SOP.
- 23.
- Seal and disconnect the collection tubing between 1 in and 2 in (2.5 cm and 5.0 cm) away from the whole blood bag. Dispose of the collection tubing safely according to your institution's SOP and/or local regulations.
- 24.
- Place the whole blood unit into a temperature-controlled environment according to your institution's SOP.
- 25.
- Pack and transport the whole blood unit to the processing laboratory according to your institution's SOP.
Post-Processing and Red Blood Cell Leukoreduction
Required Supplies:
- •
- Leukoreduction rack
- •
- Tubing sealer
- 1.
- After processing, remove the plasma bag, the plasma cryoprecipitate reduced bag, the residual leukocyte bag, and the IPU bag from the organizer and handle them according to your institution's SOP.
Note: You must rest and agitate the IPU prior to platelet pooling. For conditions for resting and agitating the IPU, see the platelet pooling set instructions for use. - 2.
- Prepare a leukoreduction rack with a head height of at least 43½ in (110 cm).
- 3.
- Remove the RBC bag from the organizer.
- 4.
- Hang the RBC bag from the leukoreduction rack so that the filter hangs vertically.
- 5.
- Open blue clamp G.
- 6.
- Break CLIKTIP H.
- 7.
- Break CLIKTIP J.
- 8.
- Drain all of the additive solution through the filter and into the whole blood bag.
- 9.
- Close blue clamp G.
- 10.
- Gently mix the additive solution with the RBC unit in the whole blood bag.
- 11.
- Hang the whole blood bag on the leukoreduction rack.
- 12.
- Ensure that the tubing is free of kinks or other obstructions.
- 13.
- Open blue clamp G and allow the mixture of red blood cells and additive solution to flow through the leukoreduction filter.
Do not squeeze the whole blood bag to increase the draining rate. Filtration is complete when the inlet side of the leukoreduction filter collapses and the red blood cells drain from the inlet side of the filter. - 14.
- Seal the RBC tubing below the leukoreduction filter and disconnect the RBC bag. Ensure that the filter remains in a vertical position while you seal the tubing, as this prevents the loss of red blood cells.
- 15.
- Dispose of the whole blood bag and filter assembly safely according to your institution's SOP and/or local regulations.
- 16.
- Seal the line for sampling segments according to your institution's SOP.
- 17.
- Store the RBC product according to your institution's SOP.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
-
3 DOSAGE FORMS AND STRENGTHS
70 mL Citrate Phosphate Dextrose (CPD) anticoagulant is a sterile solution in a PVC plasticized with DEHP bag. Each 70 mL of CPD contains: Dextrose (anhydrous) 1.624 g, Trisodium Citrate (dihydrate) 1.841 g, Citric Acid (monohydrate) 0.229 g, Sodium Dihydrogen Phosphate (dihydrate) 0.176 g, and water for injection up to 70 mL.
111 mL Additive Solution 5 (AS-5) red cell preservative solution is a sterile solution in a PVC plasticized with DEHP bag. Each 111 mL of AS-5 contains: Dextrose (anhydrous) 0.908 g, Sodium Chloride 0.973 g, Mannitol 0.583 g, Adenine 0.035 g, and water for injection up to 111 mL.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
- Single-use product. Do not reuse. This product is intended to be single use only and is not intended to be reused or re-sterilized in any manner. Terumo Blood and Cell Technologies cannot ensure the functionality or sterility of the product if it is reused or re-sterilized. Reuse of a single-use disposable set may result in:
- Product performance issues due to loss of product integrity, including but not limited to the following:
- Fluid leaks
- Parts that are warped or deformed
- Plastics that are brittle and discolored
- Filters that have reduced filtration capabilities
- Viral infections such as hepatitis or human immunodeficiency virus (HIV)
- Bacterial infections
- Cross-contamination
- Product performance issues due to loss of product integrity, including but not limited to the following:
- Inspect the packaging and the blood bag set prior to use. Do not use the set if any of the following conditions are present:
- There are tears or holes in the outer aluminum foil packaging or in the individual transparent packaging wrap.
- The tubing has severe kinks.
- The blood bag set is incorrectly assembled.
- The blood bag set is defective or damaged, or there are any leaks from the fluid-filled components of the set.
- Any clamps are closed.
- The needle cap is not in place.
- The solutions are cloudy or discolored or contain particulates.
Note: It is normal to have some condensation in the outer aluminum foil pouch and individual transparent packaging wrap due to sterilization. - The blood bag set is no longer sterile if any of the following conditions occur:
- You disconnect the sample/diversion bag before you seal the sample/diversion tubing.
- You remove blood samples before you seal the sample/diversion tubing.
- The integrity of the set is compromised for any reason.
- Do not process whole blood less than 2 hours after collection. Processing blood too soon after collection may result in incomplete RBC leukoreduction and/or a reduced platelet yield.
- Residual leukocytes are a by-product of the processing procedure and contain mostly white blood cells (WBC), with some plasma, platelets, and red blood cells (RBC). Residual leukocytes are not intended for transfusion.
- Use aseptic technique during blood collection to ensure donor safety and product quality.
- Do not vent the blood bag set.
- Single-use product. Do not reuse. This product is intended to be single use only and is not intended to be reused or re-sterilized in any manner. Terumo Blood and Cell Technologies cannot ensure the functionality or sterility of the product if it is reused or re-sterilized. Reuse of a single-use disposable set may result in:
- 6. ADVERSE REACTIONS
- 8 USE IN SPECIFIC POPULATIONS
-
11 DESCRIPTION
The Reveos SELECT set has been evaluated for use with the Reveos system.
The blood and fluid pathways of the blood bag set are steam-sterilized and are non-pyrogenic.
The blood bag set is intended for use by appropriately trained phlebotomists who collect whole blood and by blood center personnel who process whole blood using the Reveos system.
The formulas of the active ingredients are provided in Tables 1 and 2.
Table 1: CPD Active Ingredients Ingredients Molecular Formula Molecular Weight Dextrose (anhydrous) C6H12O6 180.16 g/mol Trisodium Citrate (dihydrate) C6H5Na3O72H2O 294.10 g/mol Citric Acid (monohydrate) C6H8O7H2O 210.14 g/mol Sodium Dihydrogen Phosphate (dihydrate) NaH2PO42H2O 156.01 g/mol Water for Injection H2O 18.02 g/mol Each 70 mL of CPD contains: Dextrose (anhydrous) 1.624 g, Trisodium Citrate (dihydrate) 1.841 g, Citric Acid (monohydrate) 0.229 g, Sodium Dihydrogen Phosphate (dihydrate) 0.176 g, and water for injection up to 70 mL.
Table 2: AS-5 Active Ingredients Ingredients Molecular Formula Molecular Weight Dextrose (anhydrous) C6H12O6 180.16 g/ mol Sodium Chloride NaCl 58.44 g/mol Mannitol C6H14O6 182.17 g/mol Adenine C5H5N5 135.13 g/mol Water for injection H2O 18.02 g/mol Each 111 mL of AS-5 contains: Dextrose (anhydrous) 0.908 g, Sodium Chloride 0.973 g, Mannitol 0.583 g, Adenine 0.035 g, and water for injection up to 111 mL.
The PVC plasticized with DEHP bags are not made with natural rubber latex.
The bags contain materials that have been tested to demonstrate the suitability of the containers for storing pharmaceutical solutions. The bags are nontoxic and biologically inert. The blood bag set is a closed system and is not dependent upon entry of external air during administration. The blood bag set is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
-
12 CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
CPD Mechanism of Action
CITRATE PHOSPHATE DEXTROSE acts as an extracorporeal anticoagulant by binding the free calcium in the blood. Calcium is a necessary co-factor to several steps in the clotting cascade. The following ingredients are key components of the solution:
- Citric Acid for pH regulation
- Sodium Citrate functions as an anticoagulant
- Dextrose for isotonicity
- Sodium Dihydrogen Phosphate for pH buffering
This solution has no clinical effect in transfused patients.
AS-5 Mechanism of Action
ADDITIVE SOLUTION FORMULA 5 acts to preserve and extend the shelf life of packed RBC products for later transfusion to patients. The following ingredients are key components of the solution:
- Dextrose for RBC nutrition
- Sodium Chloride for isotonicity
- Mannitol to protect RBC membranes
- Adenine to support adenosine triphosphate (ATP) levels
This solution has no clinical effect in transfused patients.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
The blood bag sets are packaged in outer aluminum foil pouches. Each outer aluminum foil pouch contains 2 blood bag sets. Each case contains 8 outer aluminum foil pouches.
CATALOG NUMBER NDC NUMBER 6FO506A0 Carton: 82906-506-16 Aluminum foil: 82906-506-02 Primary Collect Bag: 82906-506-01 STORAGE
- Long-term storage temperature: 1 °C to 30 °C
- Permitted temperature excursions:
- –20 °C to 1 °C for up to 2 weeks
- Up to 50 °C for up to 1 week
Use blood bag sets within 28 days after you open the outer aluminum foil pouch. To store unused blood bag sets, return them to the outer aluminum foil pouch and reclose the pouch with tape or a clip. Once you open the transparent packing wrap, you must use the blood bag set within 7 days, not exceeding 28 days from when you opened the outer aluminum foil pouch. Each outer aluminum foil pouch contains sachets that absorb oxygen. Dispose of the sachets and the outer aluminum foil pouch with normal waste.
- SPL UNCLASSIFIED SECTION
-
RETURN OF USED PRODUCT
If for any reason this product must be returned to Terumo BCT, Inc., a returned goods authorization (an RGA number) is required from Terumo BCT prior to shipping. Instructions for cleaning and materials, including appropriate shipping containers, proper labeling, and an RGA number, may be obtained from the Terumo BCT Quality Assurance Department. IT IS THE RESPONSIBILITY OF THE HEALTH CARE INSTITUTION TO ADEQUATELY PREPARE AND IDENTIFY THE PRODUCT FOR RETURN SHIPMENT. Please contact your local representative for information regarding returned goods and product complaints.
Tubing Specifications (non-sterile, nominal* dimensions) - *
- Note: The tubing dimensions listed on this document are nominal values and are intended for use in determining compatibility with various types of laboratory equipment. The nominal values are specified target dimensions; however, because they are based on non‑sterile tubing measurements, and due to variations in the manufacturing process, the actual dimensions may be slightly different.
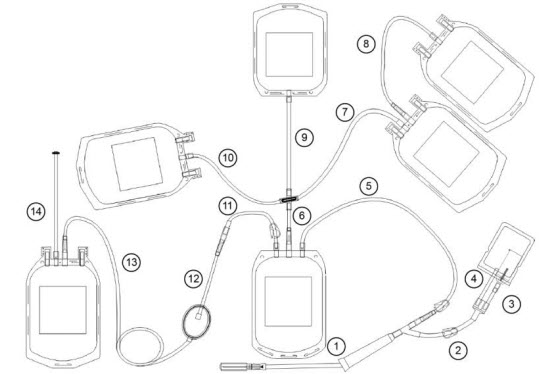
Description Primary tubing Sample tube holder/luer adapter tubing Item(s) 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 3 Wall Thickness 0.7 mm 1.3 mm Outer Diameter 4.4 mm 4.4 mm Inner Diameter 3.0 mm 1.8 mm Tubing Set Composition
- Primary tubing: Polyvinyl chloride (PVC) plasticized with Di(2-ethylhexyl)phthalate (DEHP)
- Bags: PVC plasticized with DEHP
- RBC leukoreduction filter: PVC plasticized with DEHP housing, polybutylterephthalate media
- Access needle: Stainless steel (contains cobalt)
- Needle injury protector (NIP): Polypropylene (PP)
- Sample tube holder/luer adapter: PVC/polycarbonate housing, stainless steel needle
- Clamps: Polyacetal
- Cross-connector: PVC
Not made with natural rubber latex.
- SPL UNCLASSIFIED SECTION
-
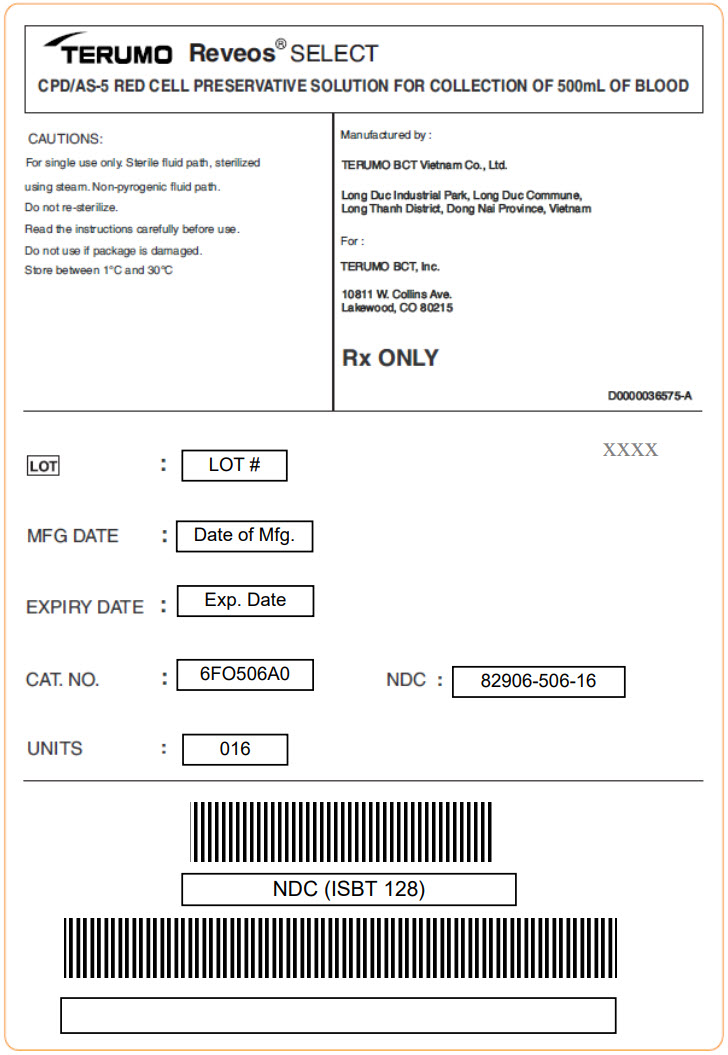
PRINCIPAL DISPLAY PANEL - 70 mL Bag Pouch Case Label
TERUMO Reveos® SELECT
CPD/AS-5 RED CELL PRESERVATIVE SOLUTION FOR COLLECTION OF 500mL OF BLOOD
CAUTIONS:
For single use only. Sterile fluid path, sterilized
using steam. Non-pyrogenic fluid path.Do not re-sterilize.
Read the instructions carefully before use.
Do not use if package is damaged.
Store between 1°C and 30°C
Manufactured by :
TERUMO BCT Vietnam Co., Ltd.
Long Duc Industrial Park, Long Duc Commune,
Long Thanh District, Dong Nai Province, VietnamFor :
TERUMO BCT, Inc.
10811 W. Collins ave.
Lakewood, CO 80215Rx ONLY
D0000036575-A
XXXX
LOT : LOT #
MFG DATE : Date of Mfg.
EXPIRY DATE : Exp. Date
CAT. NO. : 6FO506A0
UNITS : 016
NDC : 82906-506-16
NDC (ISBT 128)
-
INGREDIENTS AND APPEARANCE
REVEOS SELECT CPD/AS-5 RED CELL PRESERVATIVE FOR COLLECTION OF BLOOD
dextrose monohydrate, trisodium citrate dihydrate, anhydrous citric acid, and sodium phosphate, monobasic, unspecified form solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82906-506 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextrose Monohydrate (UNII: LX22YL083G) (Anhydrous Dextrose - UNII:5SL0G7R0OK) Dextrose Monohydrate 1.624 g in 70 mL Trisodium Citrate Dihydrate (UNII: B22547B95K) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 1.841 g in 70 mL Anhydrous Citric Acid (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) Anhydrous Citric Acid 0.229 g in 70 mL Sodium Phosphate, Monobasic, Unspecified Form (UNII: 3980JIH2SW) (PHOSPHATE ION - UNII:NK08V8K8HR, SODIUM CATION - UNII:LYR4M0NH37) Sodium Phosphate, Monobasic, Unspecified Form 0.176 g in 70 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82906-506-16 16 in 1 CASE 1 NDC:82906-506-02 2 in 1 POUCH 1 NDC:82906-506-01 70 mL in 1 BAG; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN880217 08/16/2023 Labeler - Terumo BCT Vietnam CO., Ltd. (555361198) Registrant - Terumo BCT, Ltd. (233649834) Establishment Name Address ID/FEI Business Operations Terumo BCT Vietnam CO., Ltd. 555361198 LABEL(82906-506) , ANALYSIS(82906-506) , STERILIZE(82906-506) , MANUFACTURE(82906-506)