Label: DEXAMETHASONE SODIUM PHOSPHATE solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 57319-065-01 - Packager: Phoenix Pharmaceutical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 4, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Dexamethasone Sodium Phosphate Ophthalmic Solution USP, is a sterile, topical steroid solution. The active ingredient is represented by the following structural formula:
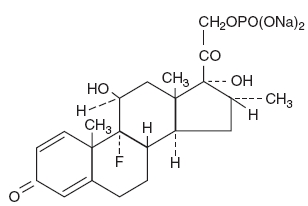
C22H28FNa2O8P
Mol. wt. 516.41
Chemical Name: 9-fluoro-11β, 17-dihydroxy-16α-methyl-21-[phosphonooxy]pregna-1, 4-diene-3,20-dione disodium salt.
Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic. Dexamethasone is a synthetic analog of naturally occurring glucocorticoids (hydrocortisone and cortisone). Dexamethasone sodium phosphate is a water soluble, inorganic ester of dexamethasone. It is approximately three thousand times more soluble at 25°C than hydrocortisone.
Each mL Contains: ACTIVE: Dexamethasone Sodium Phosphate, [equivalent to 1 mg (0.1%) Dexamethasone Phosphate]. INACTIVES: Sodium Citrate, Sodium Borate, Creatinine, Polysorbate 80, Edetate Disodium Dihydrate, Purified Water. Hydrochloric Acid may be added to adjust pH (6.6 - 7.8). PRESERVATIVES ADDED: Sodium Bisulfite 0.1%, Phenylethyl Alcohol 0.25%, Benzalkonium Chloride 0.02%.
- CLINICAL PHARMACOLOGY:
-
INDICATIONS AND USAGE:
For the treatment of the following conditions:
Ophthalmic: Steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe, such as allergic conjunctivitis, acne rosacea, superficial punctate keratitis, herpes zoster keratitis, iritis, cyclitis, selected infective conjunctivitis when the inherent hazard of steroid use is accepted to obtain an advisable diminution in edema and inflammation; corneal injury from chemical or thermal burns, or penetration of foreign bodies.
Otic: Steroid responsive inflammatory conditions of the external auditory meatus, such as allergic otitis externa, selected purulent and nonpurulent infective otitis externa when the hazard of steroid use is accepted to obtain an advisable diminution in edema and inflammation.
-
CONTRAINDICATIONS:
Epithelial herpes simplex keratitis (dendritic keratitis).
Acute infectious stages of vaccinia, varicella and many other viral diseases of the cornea and conjunctiva.
Mycobacterial infection of the eye.
Fungal diseases of ocular or auricular structures.
Hypersensitivity to any component of this product, including sulfites (see WARNINGS).
Perforation of a drum membrane.
-
WARNINGS:
Prolonged use may result in ocular hypertension and/or glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and posterior subcapsular cataract formation. Prolonged use may suppress the host response and thus increase the hazard of secondary ocular infections. In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical corticosteroids. In acute purulent conditions of the eye or ear, corticosteroids may mask infection or enhance existing infection. If these products are used for 10 days or longer, intraocular pressure should be routinely monitored even though it may be difficult in children and uncooperative patients.
Employment of corticosteroid medication in the treatment of herpes simplex other than epithelial herpes simplex keratitis, in which it is contraindicated, requires great caution; periodic slit-lamp microscopy is essential.
This product contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
-
PRECAUTIONS:
General
The possibility of persistent fungal infections of the cornea should be considered after prolonged corticosteroid dosing.
There have been reports of bacterial keratitis associated with the use of multiple dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface. (See PRECAUTIONS, Information for Patients.)
Information for Patients:
Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures.
Patients should also be instructed that ocular preparations, if handled improperly, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions. (See PRECAUTIONS, General.)
Patients should also be advised that if they develop an intercurrent ocular condition (e.g., trauma, ocular surgery or infection), they should immediately seek their physician's advice concerning the continued use of the present multidose container.
One of the preservatives in dexamethasone sodium phosphate ophthalmic solution, benzalkonium chloride, may be absorbed by soft contact lenses. Patients wearing soft contact lenses should be instructed to wait at least 15 minutes after instilling dexamethasone sodium phosphate ophthalmic solution before they insert their lenses.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of dexamethasone sodium phosphate ophthalmic solution.
Pregnancy
Pregnancy Category C. Dexamethasone has been shown to be teratogenic in mice and rabbits following topical ophthalmic application in multiples of the therapeutic dose.
In the mouse, corticosteroids produce fetal resorptions and a specific abnormality, cleft palate. In the rabbit, corticosteroids have produced fetal resorptions and multiple abnormalities involving the head, ears, limbs, palate, etc.
There are no adequate or well-controlled studies in pregnant women. Dexamethasone sodium phosphate ophthalmic solution should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the embryo or fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy should be observed carefully for signs of hypoadrenalism.
Nursing Mothers
Topically applied steroids are absorbed systemically. Therefore, because of the potential for serious adverse reactions in nursing infants from dexamethasone sodium phosphate, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS:
Glaucoma with optic nerve damage, visual acuity and field defects, posterior subcapsular cataract formation, secondary ocular infection from pathogens including herpes simplex, perforation of the globe.
Rarely, filtering blebs have been reported when topical steroids have been used following cataract surgery.
Rarely, stinging or burning may occur.
-
DOSAGE AND ADMINISTRATION:
The duration of treatment will vary with the type of lesion and may extend from a few days to several weeks, according to therapeutic response. Relapses, more common in chronic active lesions than in self-limited conditions, usually respond to retreatment.
Eye: Instill one or two drops of solution into the conjunctival sac every hour during the day and every two hours during the night as initial therapy. When a favorable response is observed, reduce dosage to one drop every four hours. Later, further reduction in dosage to one drop three or four times daily may suffice to control symptoms.
Ear: Clean the aural canal thoroughly and sponge dry. Instill the solution directly into the aural canal. A suggested initial dosage is three or four drops two or three times a day. When a favorable response is obtained, reduce dosage gradually and eventually discontinue.
If preferred, the aural canal may be packed with a gauze wick saturated with solution. Keep the wick moist with the preparation and remove from the ear after 12 to 24 hours. Treatment may be repeated as often as necessary at the discretion of the physician.
-
HOW SUPPLIED:
Dexamethasone Sodium Phosphate Ophthalmic Solution USP 0.1%, is supplied in a plastic squeeze bottle with a controlled drop tip in the following size: 5 mL - Prod. No. 04407
Storage: Store between 15°-30°C (59°-86°F).DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT.
KEEP OUT OF REACH OF CHILDREN.
Bausch & Lomb IncorporatedTampa, FL 33637
©Bausch & Lomb Incorporated
9100200 (Folded)
9100300 (Flat) - PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DEXAMETHASONE SODIUM PHOSPHATE
dexamethasone sodium phosphate solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:57319-065 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXAMETHASONE SODIUM PHOSPHATE (UNII: AI9376Y64P) (DEXAMETHASONE - UNII:7S5I7G3JQL) DEXAMETHASONE SODIUM PHOSPHATE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CREATININE (UNII: AYI8EX34EU) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) PHENETHYL ALCOHOL (UNII: ML9LGA7468) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SODIUM BISULFITE (UNII: TZX5469Z6I) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57319-065-01 1 in 1 CARTON 1 5 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040069 07/26/1996 Labeler - Phoenix Pharmaceutical, Inc. (150711039) Registrant - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Bausch & Lomb Incorporated 807927397 MANUFACTURE


