Label: AEROCOOL COLD SPRAY-
- NHRIC Code(s): 55305-112-10
- Packager: Aero Healthcare, LLC.
- Category: OTC MEDICAL DEVICE LABEL
- DEA Schedule: None
- Marketing Status: Premarket Notification
Drug Label Information
Updated August 20, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
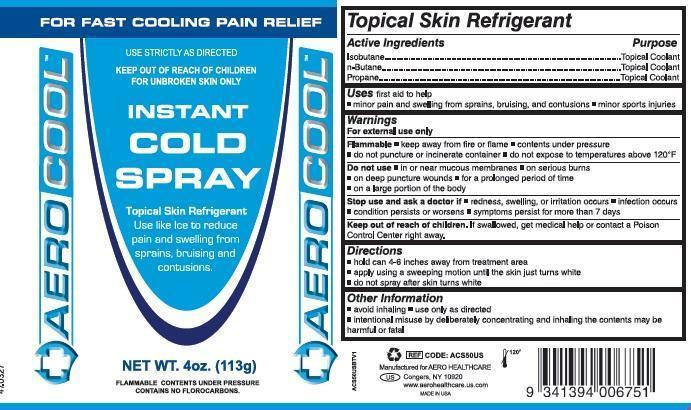
- Active Ingredients
- Purpose
- Uses
- Warnings
- Do not use
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other information
- Principal Display
-
INGREDIENTS AND APPEARANCE
AEROCOOL COLD SPRAY
refrigerant, topical (vapocoolant)Product Information Product Type OTC MEDICAL DEVICE Item Code (Source) NHRIC:55305-112 Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ISOBUTANE (UNII: BXR49TP611) INGR BUTANE (UNII: 6LV4FOR43R) INGR PROPANE (UNII: T75W9911L6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:55305-112-10 113 g in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K033720 08/20/2014 Labeler - Aero Healthcare, LLC. (008186174)