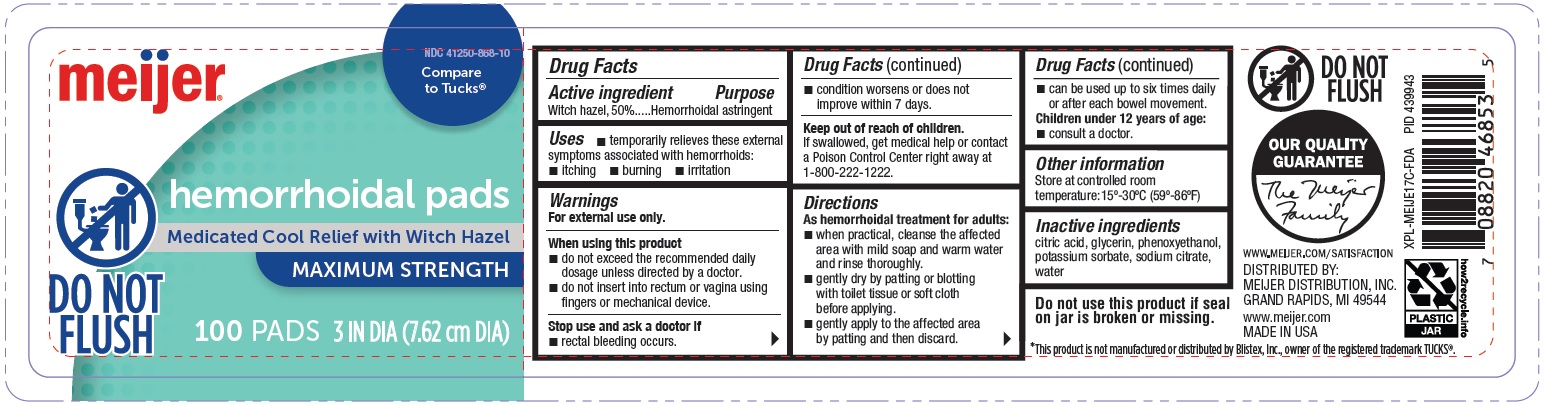
Label: HEMORRHOIDAL PADS- witch hazel patch
- NDC Code(s): 41250-868-10
- Packager: Meijer Distribution Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external rectal use only.
When using this product
- do not exceed the recommended daily dosage unless directed by a doctor.
- do not insert into rectum or vagina using fingers or mechanical device.
-
Directions
As hemorrhoidal treatment for adults:
- when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly.
- gently dry by patting or blotting with toilet tissue or soft cloth before applying.
- gently apply to the affected area by patting and then discard.
- can be used up to six times daily or after each bowel movement.
Children under 12 years of age
- consult a doctor.
- Other Information
- Inactive Ingredients
- OTHER SAFETY INFORMATION
- SPL UNCLASSIFIED SECTION
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HEMORRHOIDAL PADS
witch hazel patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-868 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WITCH HAZEL (UNII: 101I4J0U34) (WITCH HAZEL - UNII:101I4J0U34) WITCH HAZEL 500 mg in 1000 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-868-10 100 in 1 BOX 10/02/2019 1 500 mg in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 06/28/2018 Labeler - Meijer Distribution Inc. (006959555) Registrant - Meijer Distribution Inc. (006959555) Establishment Name Address ID/FEI Business Operations U. S. Nonwovens Corp 080453184 manufacture(41250-868)