Label: GLYBURIDE tablet
-
NDC Code(s):
0093-9364-01,
0093-9364-05,
0093-9364-10,
0093-9433-01, view more0093-9433-05, 0093-9477-53
- Packager: TEVA Pharmaceuticals USA Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 1, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Glyburide is an oral blood-glucose-lowering drug of the sulfonylurea class. It is a white, crystalline compound, formulated as tablets of 1.25 mg, 2.5 mg, and 5 mg strengths for oral administration. Glyburide tablets USP contain the active ingredient glyburide and the following inactive ingredients: dibasic calcium phosphate USP, magnesium stearate NF, microcrystalline cellulose NF, sodium alginate NF, talc USP. Glyburide 2.5 mg tablets USP also contain FD&C Red #40 Aluminum Lake. Glyburide 5 mg tablets USP also contain FD&C Blue #1 Aluminum Lake. Chemically, Glyburide is identified as 1-[[p-[2-(5-Chloro-o-anisamido)ethyl]phenyl]sulfonyl]-3-cyclohexylurea.
The CAS Registry Number is 10238-21-8.
The structural formula is:
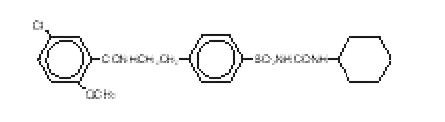
The molecular weight is 493.99. The aqueous solubility of Glyburide increases with pH as a result of salt formation.
-
CLINICAL PHARMACOLOGY
Glyburide appears to lower the blood glucose acutely by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets. The mechanism by which Glyburide lowers blood glucose during long-term administration has not been clearly established.
With chronic administration in Type II diabetic patients, the blood glucose lowering effect persists despite a gradual decline in the insulin secretory response to the drug. Extrapancreatic effects may play a part in the mechanism of action of oral sulfonylurea hypoglycemic drugs.
In addition to its blood glucose lowering actions, Glyburide produces a mild diuresis by enhancement of renal free water clearance. Clinical experience to date indicates an extremely low incidence of disulfiram-like reactions in patients while taking Glyburide.
Pharmacokinetics
Single-dose studies with Glyburide in normal subjects demonstrate significant absorption within 1 hour, peak drug levels at about 4 hours, and low but detectable levels at 24 hours. Mean serum levels of glyburide, as reflected by areas under the serum concentration-time curve, increase in proportion to corresponding increases in dose. Multiple-dose studies with Glyburide in diabetic patients demonstrate drug level concentration-time curves similar to single-dose studies, indicating no build-up of drug in tissue depots. The decrease of glyburide in the serum of normal healthy individuals is biphasic, the terminal half-life being about 10 hours. In single-dose studies in fasting normal subjects, the degree and duration of blood glucose lowering is proportional to the dose administered and to the area under the drug level concentration-time curve. The blood glucose lowering effect persists for 24 hours following single morning doses in non-fasting diabetic patients. Under conditions of repeated administration in diabetic patients, however, there is no reliable correlation between blood drug levels and fasting blood glucose levels. A one-year study of diabetic patients treated with Glyburide showed no reliable correlation between administered dose and serum drug level.
The major metabolite of Glyburide is the 4-trans-hydroxy derivative. A second metabolite, the 3-cis-hydroxy derivative, also occurs. These metabolites contribute no significant hypoglycemic action since they are only weakly active (1/400th and 1/40th, respectively, as glyburide) in rabbits.
Glyburide is excreted as metabolites in the bile and urine, approximately 50% by each route. This dual excretory pathway is qualitatively different from that of other sulfonylureas, which are excreted primarily in the urine.
Sulfonylurea drugs are extensively bound to serum proteins. Displacement from protein binding sites by other drugs may lead to enhanced hypoglycemic action. In vitro, the protein binding exhibited by Glyburide is predominantly non-ionic, whereas that of other sulfonylureas (chlorpropamide, tolbutamide, tolazamide) is predominantly ionic. Acidic drugs such as phenylbutazone, warfarin, and salicylates displace the ionic-binding sulfonylureas from serum proteins to a far greater extent than the non-ionic binding Glyburide. It has not been shown that this difference in protein binding will result in fewer drug-drug interactions with Glyburide in clinical use.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITY
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups (Diabetes 19 (supp. 2): 747–830, 1970).
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2-1/2 times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of Glyburide and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
Persons allergic to other sulfonamide derivatives may develop an allergic reaction to glyburide as well.
-
PRECAUTIONS
General
Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with Glyburide or any other anti-diabetic drug.
Hypoglycemia
All sulfonylurea drugs are capable of producing severe hypoglycemia. Proper patient selection, dosage, and instructions are important to avoid hypoglycemic episodes. Severe renal or hepatic insufficiency may cause elevated blood levels of Glyburide and the latter may also diminish gluconeogenic capacity, both of which increase the risk of serious, prolonged hypoglycemic reactions. Elderly, debilitated or malnourished patients, and those with adrenal or pituitary insufficiency are particularly susceptible to the hypoglycemic action of glucose-lowering drugs. Hypoglycemia may be difficult to recognize in patients with autonomic neuropathy, the elderly, and in people who are taking beta-adrenergic blocking drugs or other sympatholytic agents.
Hypoglycemia is more likely to occur when caloric intake is deficient, after severe or prolonged exercise, when alcohol is ingested, or when more than one glucose-lowering drug is used. Loss of control of blood glucose: When a patient stabilized on any diabetic regimen is exposed to stress such as fever, trauma, infection, or surgery, a loss of control may occur. At such times, it may be necessary to discontinue Glyburide and administer insulin.
The effectiveness of any oral hypoglycemic drug, including Glyburide, in lowering blood glucose to a desired level decreases in many patients over a period of time, which may be due to progression of the severity of the diabetes or to diminished responsiveness to the drug. This phenomenon is known as secondary failure, to distinguish it from primary failure in which the drug is ineffective in an individual patient when first given.
Hemolytic Anemia
Treatment of patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency with sulfonylurea agents can lead to hemolytic anemia. Because Glyburide belongs to the class of sulfonylurea agents, caution should be used in patients with G6PD deficiency and a non-sulfonylurea alternative should be considered. In postmarketing reports, hemolytic anemia has also been reported in patients who did not have known G6PD deficiency.
Information for Patients
Patients should be informed of the potential risks and advantages of Glyburide and of alternative modes of therapy. They should also be informed about the importance of adherence to dietary instructions, of a regular exercise program, and of regular testing of blood glucose.
The risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and responsible family members. Primary and secondary failure should also be explained.
Laboratory Tests
Periodic fasting blood glucose measurements should be performed to monitor therapeutic response. A glycosylated hemoglobin determination should also be performed periodically.
Drug Interactions
The hypoglycemic action of sulfonylureas may be potentiated by certain drugs including nonsteroidal anti-inflammatory agents, ACE inhibitors, disopyramide, fluoxetine, clarithromycin, and other drugs that are highly protein bound, salicylates, sulfonamides, chloramphenicol, probenecid, monoamine oxidase inhibitors, and beta adrenergic blocking agents. When such drugs are administered to a patient receiving Glyburide, the patient should be observed closely for hypoglycemia. When such drugs are withdrawn from a patient receiving Glyburide, the patient should be observed closely for loss of control.
An increased incidence of elevated liver enzymes was observed in patients receiving glyburide concomitantly with bosentan. Therefore concomitant administration of glyburide and bosentan is contraindicated (see CONTRAINDICATIONS).
A potential interaction between oral miconazole and oral hypoglycemic agents leading to severe hypoglycemia has been reported. Whether this interaction also occurs with the intravenous, topical or vaginal preparations of miconazole is not known.
A possible interaction between glyburide and fluoroquinolone antibiotics has been reported resulting in a potentiation of the hypoglycemic action of glyburide. The mechanism for this interaction is not known.
Possible interactions between glyburide and coumarin derivatives have been reported that may either potentiate or weaken the effects of coumarin derivatives. The mechanism of these interactions is not known.
Rifampin may worsen glucose control of glyburide because rifampin can significantly induce metabolic isozymes of glyburide such as CYP2C9 and 3A4.
Certain drugs tend to produce hyperglycemia and may lead to loss of control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are administered to a patient receiving Glyburide, the patient should be closely observed for loss of control. When such drugs are withdrawn from a patient receiving Glyburide, the patient should be observed closely for hypoglycemia.
Glyburide may increase cyclosporine plasma concentration and potentially lead to its increased toxicity. Monitoring and dosage adjustment of cyclosporine are therefore recommended when both drugs are coadministered.
Colesevelam
Concomitant administration of colesevelam and glyburide resulted in reductions in glyburide AUC and Cmax of 32% and 47%, respectively. When glyburide was administered 1 hour before colesevelam, the reductions in glyburide AUC and Cmax were 20% and 15%, respectively, and not significantly changed (-7% and 4%, respectively) when administered 4 hours before colesevelam. Therefore, glyburide should be administered at least 4 hours prior to colesevelam.
Glyburide is mainly metabolized by CYP 2C9 and to a lesser extent by CYP 3A4. There is a potential for drug-drug interaction when glyburide is coadministered with inducers or inhibitors of CYP 2C9, which should be taken into account when considering concomitant therapy.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Glyburide is non-mutagenic when studied in the Salmonella microsome test (Ames test) and in the DNA damage/alkaline elution assay. Studies in rats at doses up to 300 mg/kg/day for 18 months showed no carcinogenic effects.
No drug related effects were noted in any of the criteria evaluated in the two year oncogenicity study of glyburide in mice.
Pregnancy
Teratogenic Effects
Glyburide has been shown to affect the maturation of the long bones (humerus and femur) in rat pups when given in doses 6250 times the maximum recommended human dose. These effects, which were seen during the period of lactation and not during organogenesis, are a shortening of the bones with effects to various structures of the long bones, especially in humerus and femur.
There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Glyburide should be used during pregnancy only if the potential benefit justifies the risk to the fetus. Because recent information suggests that abnormal blood glucose levels during pregnancy are associated with a higher incidence of congenital abnormalities, many experts recommend that insulin be used during pregnancy to maintain blood glucose levels as close to normal as possible.
Nonteratogenic Effects
Prolonged severe hypoglycemia (4 to 10 days) has been reported in neonates born to mothers who were receiving a sulfonylurea drug at the time of delivery. This has been reported more frequently with the use of agents with prolonged half-lives. If Glyburide is used during pregnancy, it should be discontinued at least two weeks before the expected delivery date.
Nursing Mothers
Although it is not known whether Glyburide is excreted in human milk, some sulfonylureas are known to be excreted in human milk. Because the potential for hypoglycemia in nursing infants may exist, a decision should be made whether to discontinue nursing or to discontinue administering the drug, taking into account the importance of the drug to the mother. If Glyburide is discontinued and if diet alone is inadequate for controlling blood glucose, insulin therapy should be considered.
Geriatric Use
In US clinical studies of glyburide, 1406 of 2897 patients were ≥60 years and 515 patients were ≥70 years. Differences in safety and efficacy were not determined between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Elderly patients are particularly susceptible to hypoglycemic action of glucose-lowering drugs. Hypoglycemia may be difficult to recognize in the elderly (see PRECAUTIONS). The initial and maintenance dosing should be conservative to avoid hypoglycemic reactions.
In three published studies of 20 to 51 subjects each, mixed results were seen in comparing the pharmacokinetics of glyburide in elderly versus younger subjects. However, observed pharmacodynamic differences indicate the necessity for dosage titration to a specified therapeutic response.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
In elderly, debilitated, or malnourished patients, or in patients with renal or hepatic insufficiency, the initial dosing, dose increments, and maintenance dosage should be conservative to avoid hypoglycemic reactions. Hypoglycemia may be difficult to recognize in the elderly and in people who are taking beta-adrenergic blocking drugs or other sympatholytic agents. (See PRECAUTIONS, General; and DOSAGE AND ADMINISTRATION.)
-
ADVERSE REACTIONS
Gastrointestinal Reactions
Cholestatic jaundice and hepatitis may occur rarely which may progress to liver failure; Glyburide should be discontinued if this occurs. Liver function abnormalities, including isolated transaminase elevations, have been reported. Gastrointestinal disturbances, e.g., nausea, epigastric fullness, and heartburn, are the most common reactions and occur in 1.8% of treated patients. They tend to be dose-related and may disappear when dosage is reduced.
Dermatologic Reactions
Allergic skin reactions, e.g., pruritus, erythema, urticaria, and morbilliform or maculopapular eruptions, occur in 1.5% of treated patients. These may be transient and may disappear despite continued use of Glyburide. Bullous reactions, erythema multiforme, and exfoliative dermatitis, have been reported. If skin reactions persist, the drug should be discontinued.
Porphyria cutanea tarda and photosensitivity reactions have been reported with sulfonylureas.
Hematologic Reactions
Leukopenia, agranulocytosis, thrombocytopenia, which occasionally may present as purpura, hemolytic anemia, aplastic anemia, and pancytopenia have been reported with sulfonylureas.
Metabolic Reactions
Hepatic porphyria reactions have been reported with sulfonylureas; however, these have not been reported with Glyburide. Disulfiram-like reactions have been reported very rarely with Glyburide. Cases of hyponatremia have been reported with glyburide and all other sulfonylureas, most often in patients who are on other medications or have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone. The syndrome of inappropriate antidiuretic hormone (SIADH) secretion has been reported with certain other sulfonylureas, and it has been suggested that these sulfonylureas may augment the peripheral (antidiuretic) action of ADH and/or increase release of ADH. Glyburide can cause weight gain.
Other Reactions
Changes in accommodation and/or blurred vision have been reported with glyburide and other sulfonylureas. These are thought to be related to fluctuation in glucose levels.
In addition to dermatologic reactions, allergic reactions such as angioedema, arthralgia, myalgia and vasculitis have been reported.
-
OVERDOSAGE
Overdosage of sulfonylureas, including Glyburide, can produce hypoglycemia. Mild hypoglycemic symptoms without loss of consciousness or neurologic findings should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring should continue until the physician is assured that the patient is out of danger. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given a rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of a more dilute (10%) glucose solution at a rate that will maintain the blood glucose at a level above 100 mg/dL. Patients should be closely monitored for a minimum of 24 to 48 hours, since hypoglycemia may recur after apparent clinical recovery.
-
DOSAGE AND ADMINISTRATION
There is no fixed dosage regimen for the management of diabetes mellitus with Glyburide or any other hypoglycemic agent. The patient's fasting blood glucose must be measured periodically to determine the minimum effective dose for the patient; to detect primary failure, i.e., inadequate lowering of blood glucose at the maximum recommended dose of medication; and to detect secondary failure, i.e., loss of adequate blood glucose lowering response after an initial period of effectiveness. Periodic glycosylated hemoglobin determinations should be performed.
Short-term administration of Glyburide may be sufficient during periods of transient loss of control in patients usually controlled well on diet.
1. Usual Starting Dose
The usual starting dose of Glyburide as initial therapy is 2.5 to 5 mg daily, administered with breakfast or the first main meal. Those patients who may be more sensitive to hypoglycemic drugs should be started at 1.25 mg daily. (See PRECAUTIONS Section for patients at increased risk). Failure to follow an appropriate dosage regimen may precipitate hypoglycemia. Patients who do not adhere to their prescribed dietary and drug regimen are more prone to exhibit unsatisfactory response to therapy.
Transfer of patients from other oral antidiabetic regimens to Glyburide should be done conservatively and the initial daily dose should be 2.5 to 5 mg. When transferring patients from oral hypoglycemic agents other than chlorpropamide, to Glyburide, no transition period and no initial priming dose is necessary. When transferring patients from chlorpropamide, particular care should be exercised during the first two weeks because the prolonged retention of chlorpropamide in the body and subsequent overlapping drug effects may provoke hypoglycemia.
Bioavailability studies have demonstrated that Glynase®1 PresTab®1 Tablets 3 mg are not bioequivalent to Glyburide tablets USP 5 mg. Therefore, these products are not substitutable and patients should be retitrated if transferred.
Some Type II diabetic patients being treated with insulin may respond satisfactorily to Glyburide. If the insulin dose is less than 20 units daily, substitution of Glyburide 2.5 to 5 mg as a single daily dose may be tried. If the insulin dose is between 20 and 40 units daily, the patient may be placed directly on Glyburide 5 mg daily as a single dose. If the insulin dose is more than 40 units daily, a transition period is required for conversion to Glyburide. In these patients, insulin dosage is decreased by 50% and Glyburide 5 mg daily is started. Please refer to Usual Maintenance Dose for further explanation.
When colesevelam is coadministered with glyburide, maximum plasma concentration and total exposure to glyburide is reduced. Therefore, glyburide should be administered at least 4 hours prior to colesevelam.
- 1
- Trademarks of their respective owners, not affiliated with sanofi-aventis.
2. Usual Maintenance Dose
The usual maintenance dose is in the range of 1.25 to 20 mg daily, which may be given as a single dose or in divided doses (See Dosage Interval Section). Dosage increases should be made in increments of no more than 2.5 mg at weekly intervals based upon the patient's blood glucose response.
No exact dosage relationship exists between Glyburide and the other oral hypoglycemic agents. Although patients may be transferred from the maximum dose of other sulfonylureas, the maximum starting dose of 5 mg of Glyburide should be observed. A maintenance dose of 5 mg Glyburide provides approximately the same degree of blood glucose control as 250 to 375 mg chlorpropamide, 250 to 375 mg tolazamide, 500 to 750 mg acetohexamide, or 1000 to 1500 mg tolbutamide.
When transferring patients receiving more than 40 units of insulin daily, they may be started on a daily dose of Glyburide 5 mg concomitantly with a 50% reduction in insulin dose. Progressive withdrawal of insulin and increase of Glyburide in increments of 1.25 to 2.5 mg every 2 to 10 days is then carried out. During this conversion period when both insulin and Glyburide are being used, hypoglycemia may rarely occur. During insulin withdrawal, patients should self-test their blood for glucose and their urine for acetone at least 3 times daily and report results to their physician. Self-testing of urinary glucose is a less desirable alternative. The appearance of persistent acetonuria with glycosuria indicates that the patient is a Type I diabetic who requires insulin therapy.
4. Dosage Interval
Once-a-day therapy is usually satisfactory, based upon usual meal patterns and a 10 hour half-life of Glyburide. Some patients, particularly those receiving more than 10 mg daily, may have a more satisfactory response with twice-a-day dosage.
In elderly patients, debilitated or malnourished patients, and patients with impaired renal or hepatic function, the initial and maintenance dosing should be conservative to avoid hypoglycemic reactions. (See PRECAUTIONS Section.)
-
HOW SUPPLIED
Glyburide tablets USP are available in the following strengths and package sizes:
1.25 mg (white to off-white, capsule-shaped, flat faced, beveled edge tablet debossed "GLYBUR" on one side and a score line on the other side).
Bottles of 50 (NDC 0093-9477-53)2.5 mg (pink, capsule-shaped, flat faced, beveled edge tablet debossed "GLYBUR" on one side and a score line on the other side).
Bottles of 100 (NDC 0093-9433-01)
Bottles of 500 (NDC 0093-9433-05)5 mg (blue, capsule-shaped, flat faced, beveled edge tablet debossed "GLYBUR" on one side and a score line on the other side).
Bottles of 100 (NDC 0093-9364-01)
Bottles of 500 (NDC 0093-9364-05)
Bottles of 1000 (NDC 0093-9364-10) - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1.25 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 2.5 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
GLYBURIDE
glyburide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0093-9477 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYBURIDE (UNII: SX6K58TVWC) (GLYBURIDE - UNII:SX6K58TVWC) GLYBURIDE 1.25 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM PALMITOSTEARATE (UNII: R4OXA9G5BV) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM ALGINATE (UNII: C269C4G2ZQ) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE (white to off-white) Score 2 pieces Shape OVAL (CAPSULE-SHAPED) Size 13mm Flavor Imprint Code GLYBUR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0093-9477-53 50 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA AUTHORIZED GENERIC NDA017532 05/01/1984 GLYBURIDE
glyburide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0093-9433 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYBURIDE (UNII: SX6K58TVWC) (GLYBURIDE - UNII:SX6K58TVWC) GLYBURIDE 2.5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM PALMITOSTEARATE (UNII: R4OXA9G5BV) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM ALGINATE (UNII: C269C4G2ZQ) TALC (UNII: 7SEV7J4R1U) FD&C RED NO. 40 (UNII: WZB9127XOA) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color PINK Score 2 pieces Shape OVAL (CAPSULE-SHAPED) Size 13mm Flavor Imprint Code GLYBUR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0093-9433-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/1984 2 NDC:0093-9433-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA AUTHORIZED GENERIC NDA017532 05/01/1984 GLYBURIDE
glyburide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0093-9364 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYBURIDE (UNII: SX6K58TVWC) (GLYBURIDE - UNII:SX6K58TVWC) GLYBURIDE 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM PALMITOSTEARATE (UNII: R4OXA9G5BV) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM ALGINATE (UNII: C269C4G2ZQ) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color BLUE Score 2 pieces Shape OVAL (CAPSULE-SHAPED) Size 13mm Flavor Imprint Code GLYBUR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0093-9364-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/1984 2 NDC:0093-9364-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/1984 3 NDC:0093-9364-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA AUTHORIZED GENERIC NDA017532 05/01/1984 Labeler - TEVA Pharmaceuticals USA Inc (001627975) Registrant - Sanofi-Aventis U.S. LLC (824676584) Establishment Name Address ID/FEI Business Operations Reed-Lane, Inc. 001819879 LABEL(0093-9364, 0093-9433, 0093-9477) , PACK(0093-9364, 0093-9433, 0093-9477) Establishment Name Address ID/FEI Business Operations OPELLA HEALTHCARE INTERNATIONAL SAS 275857012 ANALYSIS(0093-9364, 0093-9433, 0093-9477) , MANUFACTURE(0093-9364, 0093-9433, 0093-9477) Establishment Name Address ID/FEI Business Operations Zentiva Private Limited (Ankleshwar) 918593737 API MANUFACTURE(0093-9364, 0093-9433, 0093-9477)






