Label: RED BETTER SPOT CORRECTOR NON-DRYING ACNE BALM- salicylic acid, sulfur cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 69774-944-02 - Packager: SKIN SCIENCES LABORATORY INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 29, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
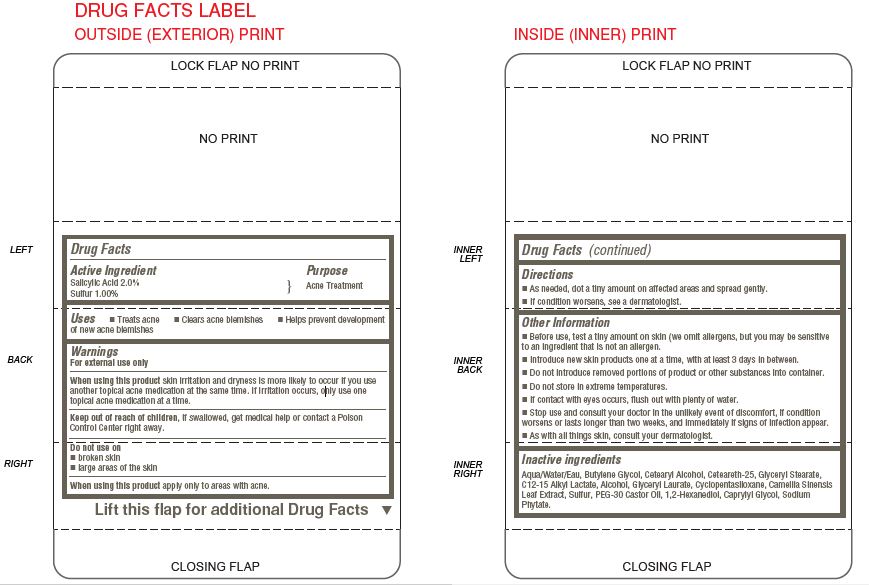
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
When using this product skin irritation and dryness is more likely to occur if you use
another topical acne medication at the same time. If irritation occurs, only use one
topical acne medication at a time.
Keep out of reach of children, if swallowed, get medical help or contact a Poison
Control Center right away.Do not use on
broken skin
large areas of the skin
When using this product apply only to areas with acne. - DOSAGE & ADMINISTRATION
-
OTHER SAFETY INFORMATION
Other Information
Before use, test a tiny amount on skin (we omit allergens, but you may be sensitive
to an ingredient that is not an allergen.
Introduce new skin products one at a time, with at least 3 days in between.
Do not introduce removed portions of product or other substances into container.
Do not store in extreme temperatures.
If contact with eyes occurs, flush out with plenty of water.
Stop use and consult your doctor in the unlikely event of discomfort, if condition
worsens or lasts longer than two weeks, and immediately if signs of infection appear.
As with all things skin, consult your dermatologist. - INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
-
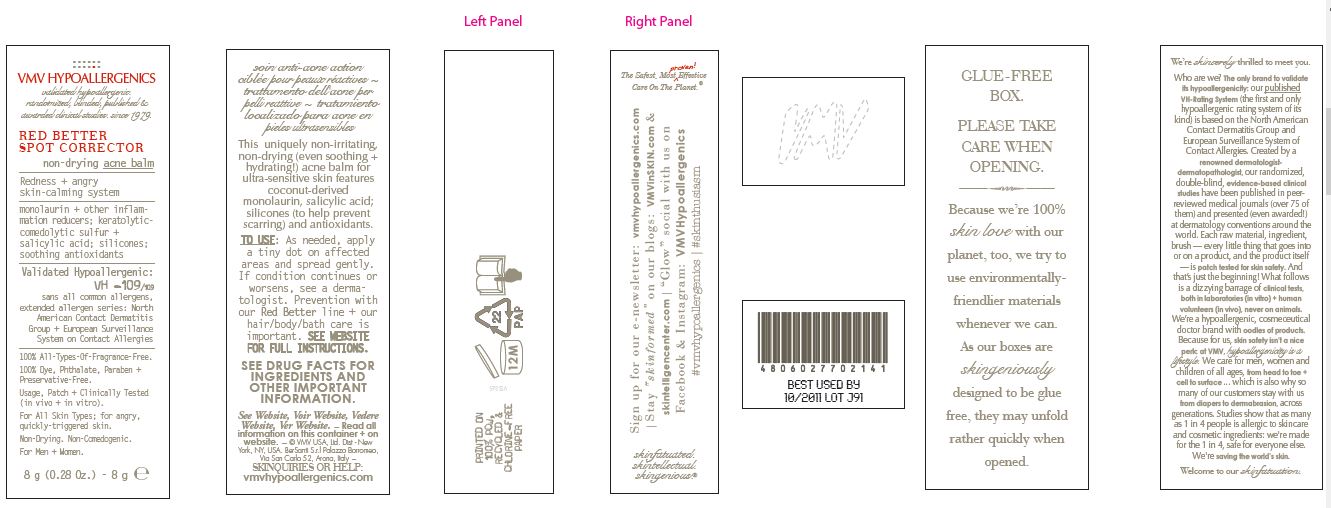
PRINCIPAL DISPLAY PANEL
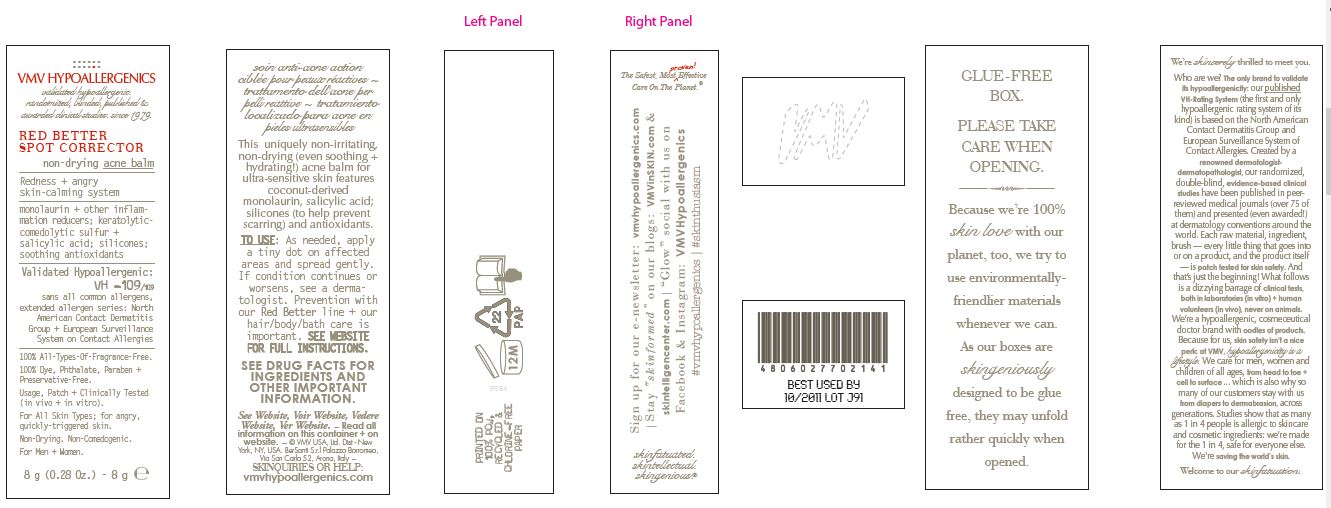
VMV HYPOALLERGENICS
validated hypoallergenic.
randomized, blinded, published &
awarded clinical studies. since 1979RED BETTER
SPOT CORRECTOR
non-drying acne balmRedness + angry
skin-calming systemmonolaurin + other inflammation reducers
keratolytic comedolytic sulfur +
salicylic acid; silicones;
soothing antioxidantsValidated
Hypoallergenic:
VH -109/109
sans all common allergens,
extended allergen series:
North American Contact Dermatitis
Group + European Surveillance
System on Contact Allergies100% All-Types-Of-Fragrance-Free.
100% Dye, Phthalate, Paraben + Preservative-Free.
Usage, Patch + Clinically-Tested (in vitro + in vivo)
For All Skin Types; for angry, quickly-triggered skin.
Non-Drying. Non-Comedogenic.
For Men + Women.
8 g (0.28 Oz.) - 8g
-
INGREDIENTS AND APPEARANCE
RED BETTER SPOT CORRECTOR NON-DRYING ACNE BALM
salicylic acid, sulfur creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69774-944 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 0.08 g in 8 g SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.16 g in 8 g Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARETH-25 (UNII: 8FA93U5T67) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHYTATE SODIUM (UNII: 88496G1ERL) WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERYL LAURATE (UNII: Y98611C087) PEG-30 CASTOR OIL (UNII: GF873K38RZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69774-944-02 1 in 1 BOX 05/30/2017 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 05/30/2017 Labeler - SKIN SCIENCES LABORATORY INC. (718777360)