Label: TOTAL EYE 3-IN-1 RENEWAL THERAPY SPF 35- titanium dioxide and zinc oxide liquid
TOTAL EYE 3-IN-1 RENEWAL THERAPY SPF 35 (FAIR)- titanium dioxide and zinc oxide liquid
TOTAL EYE 3-IN-1 RENEWAL THERAPY SPF 35 (TAN)- titanium dioxide and zinc oxide liquid
TOTAL EYE 3-IN-1 RENEWAL THERAPY SPF 35 (DEEP)- titanium dioxide and zinc oxide liquid
-
NDC Code(s):
68078-024-01,
68078-024-02,
68078-046-01,
68078-047-01, view more68078-048-01
- Packager: Colorescience
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
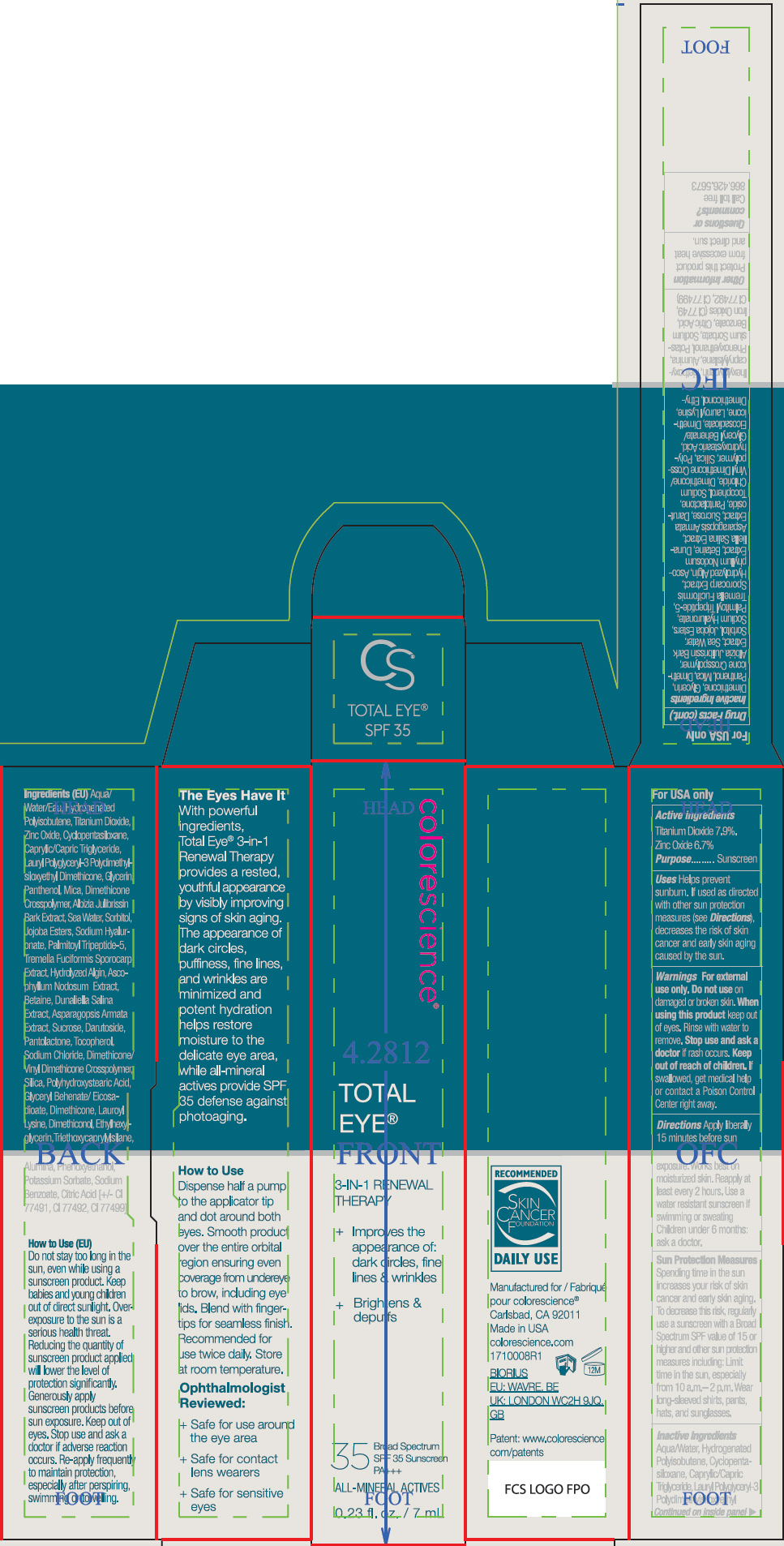
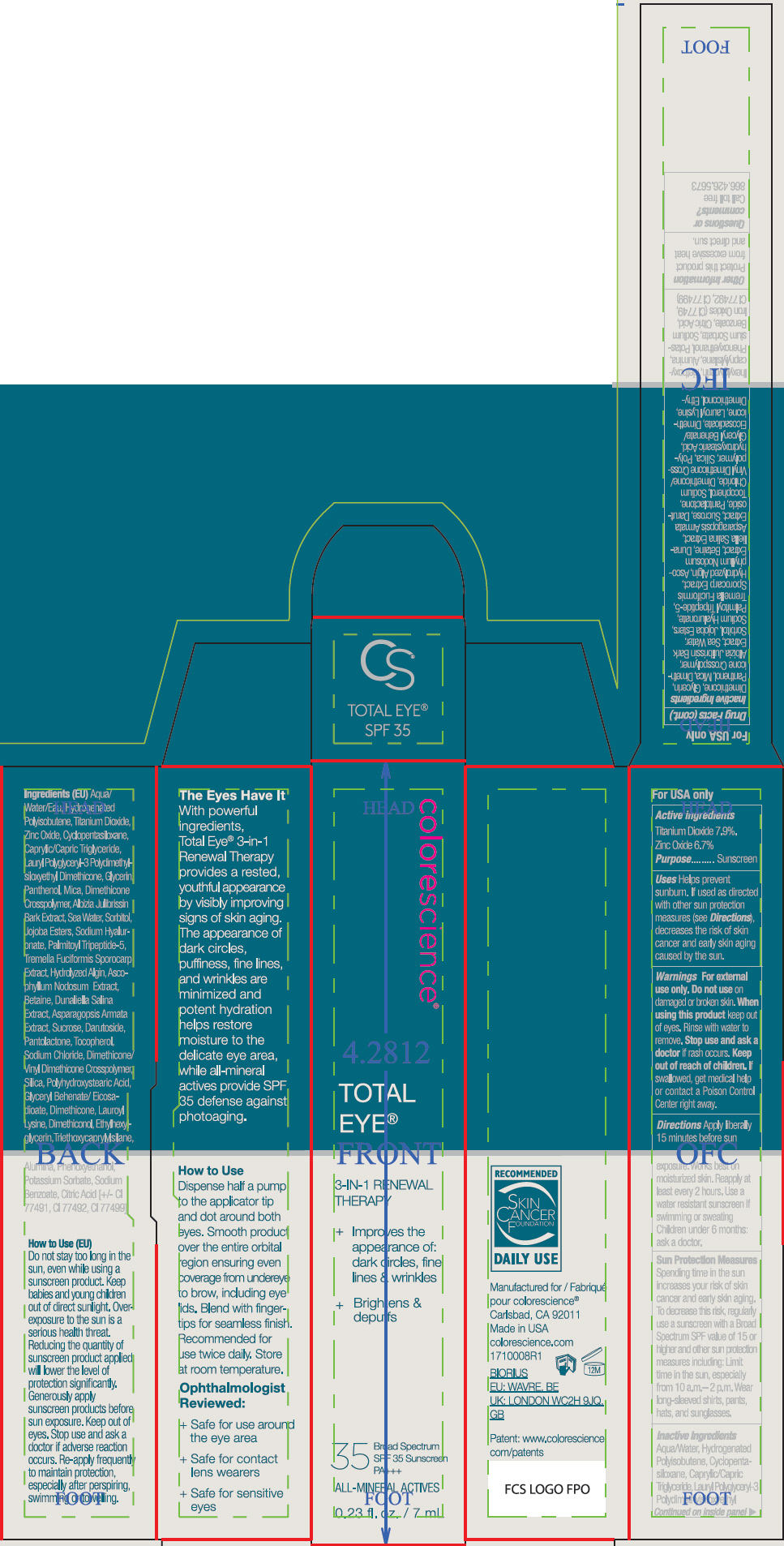
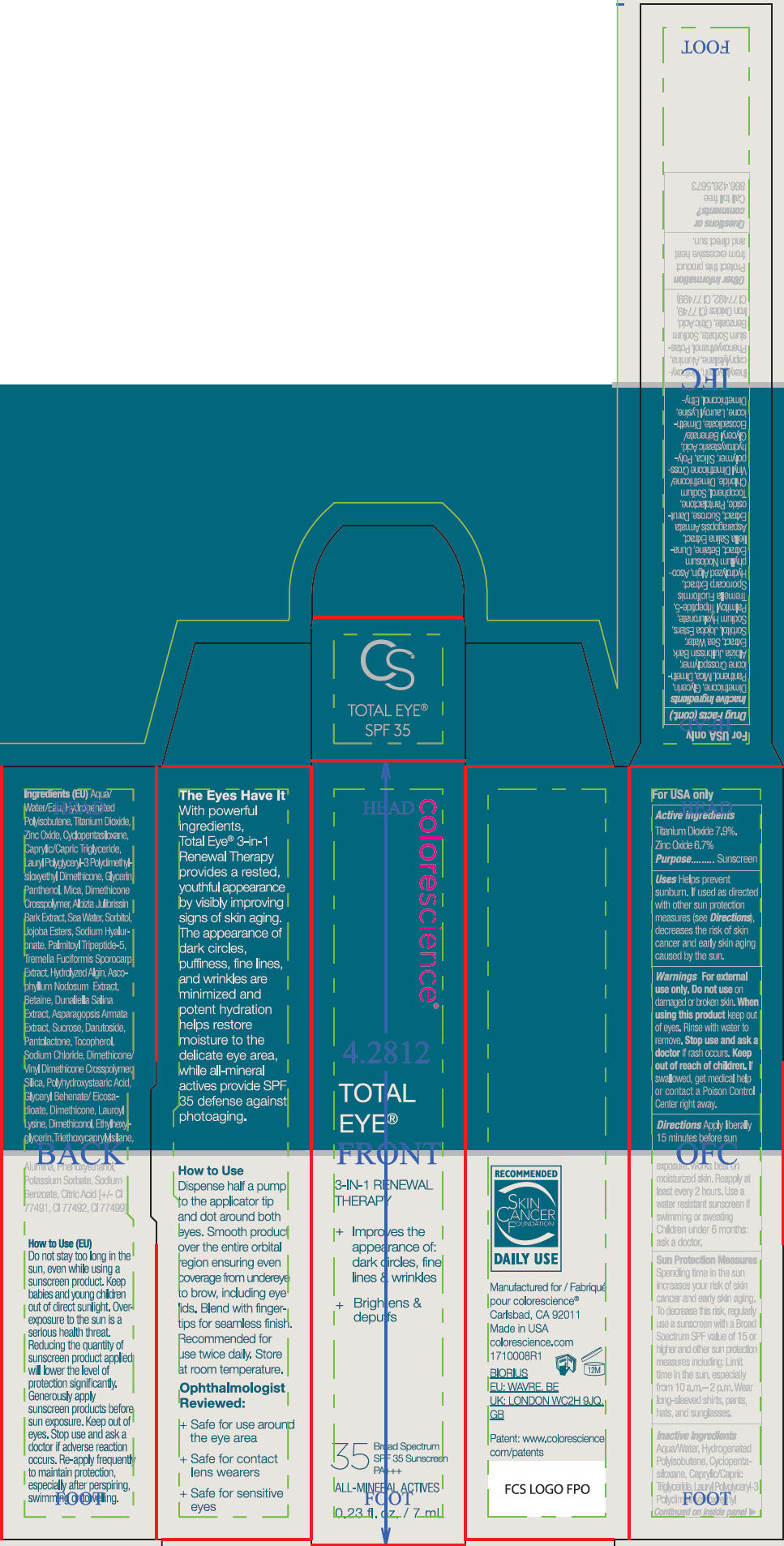
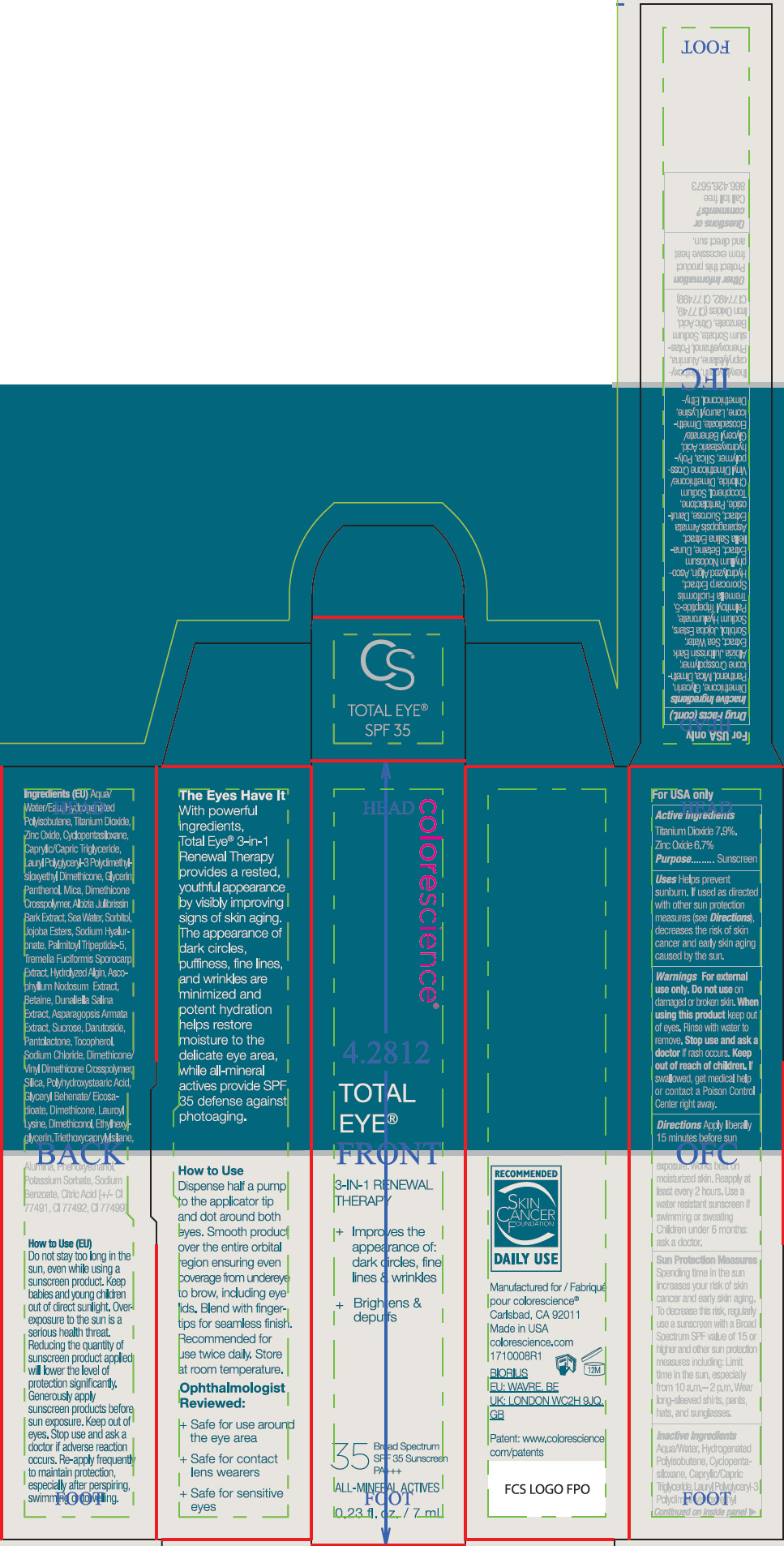
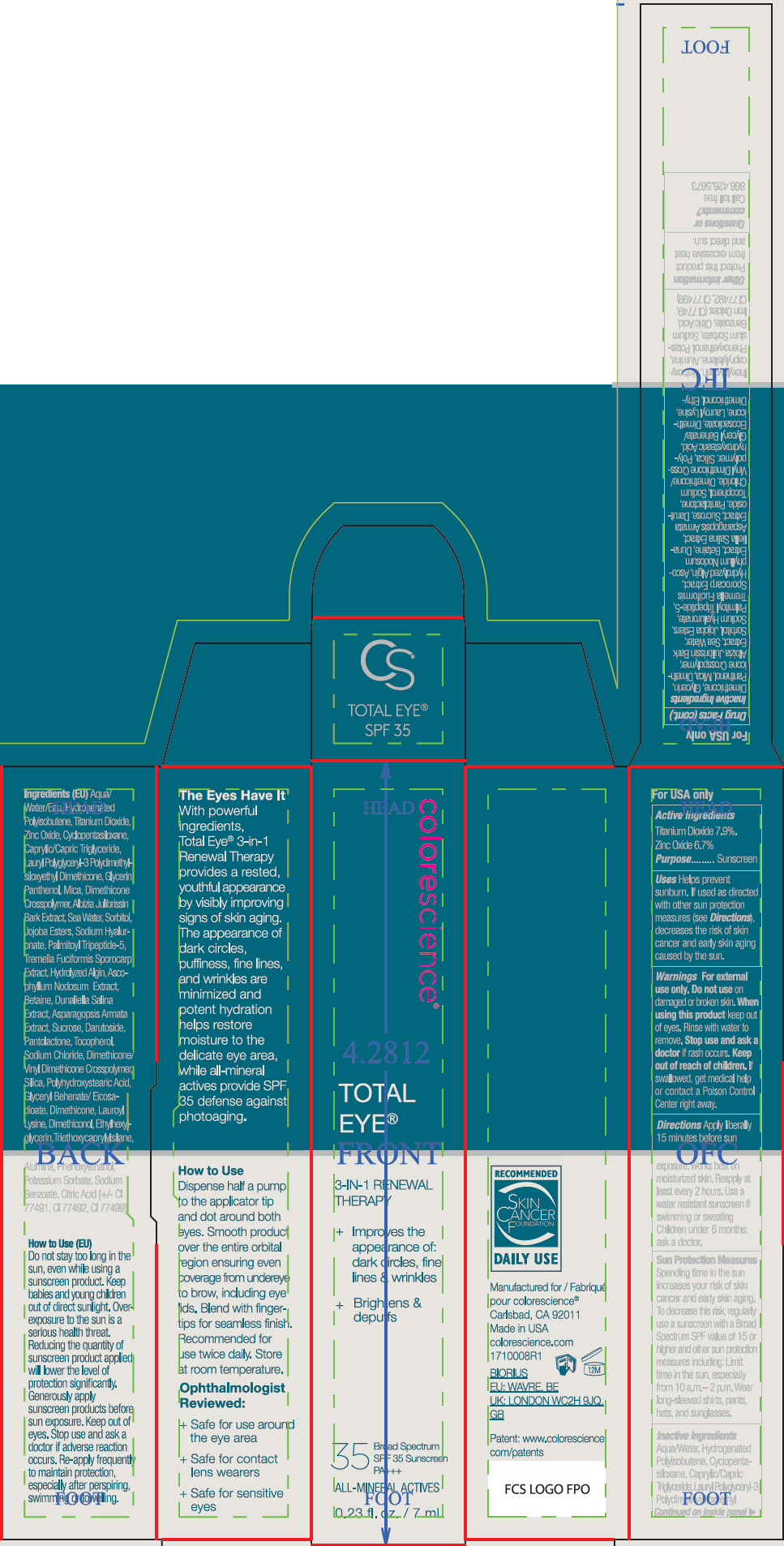
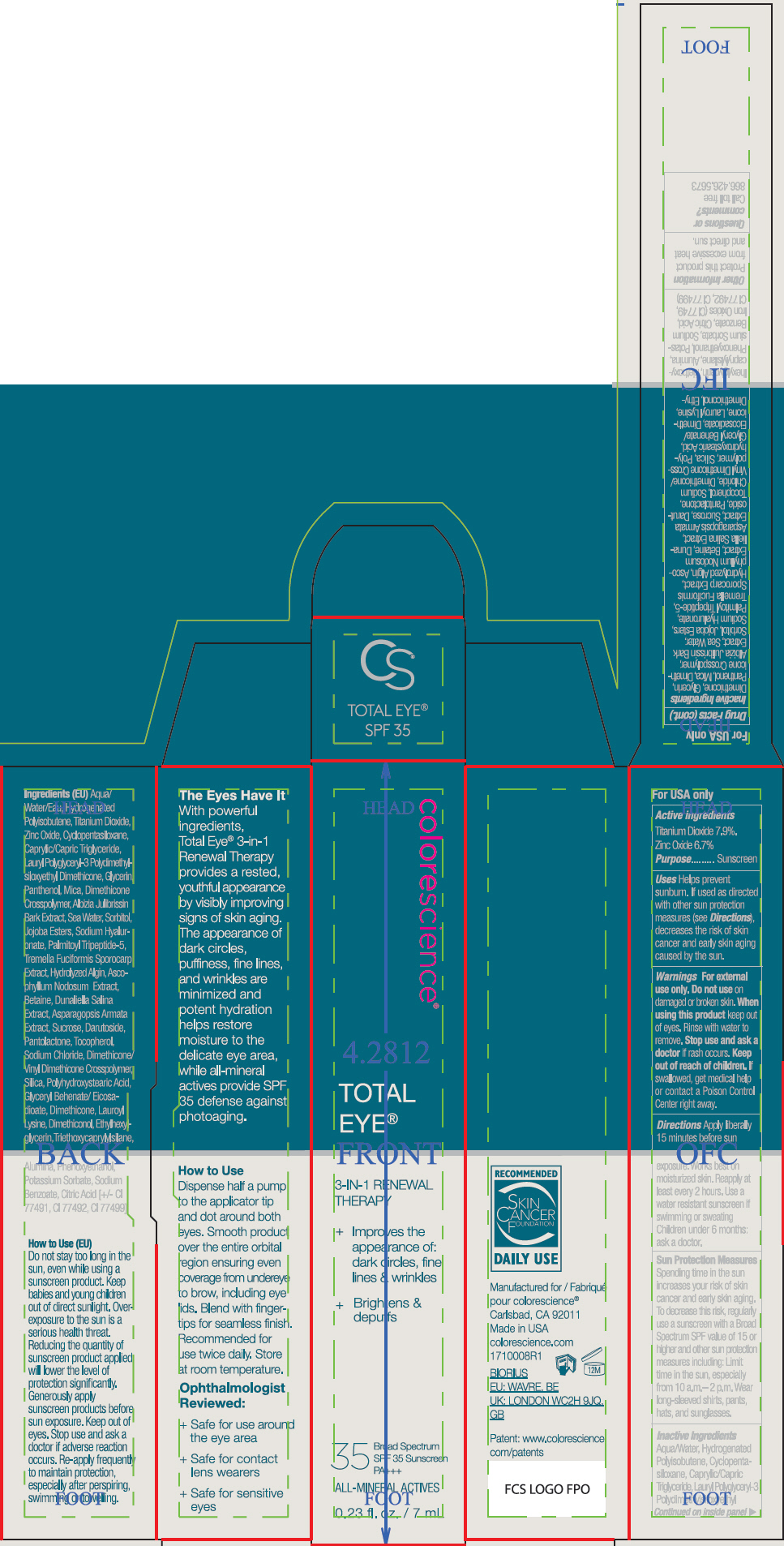
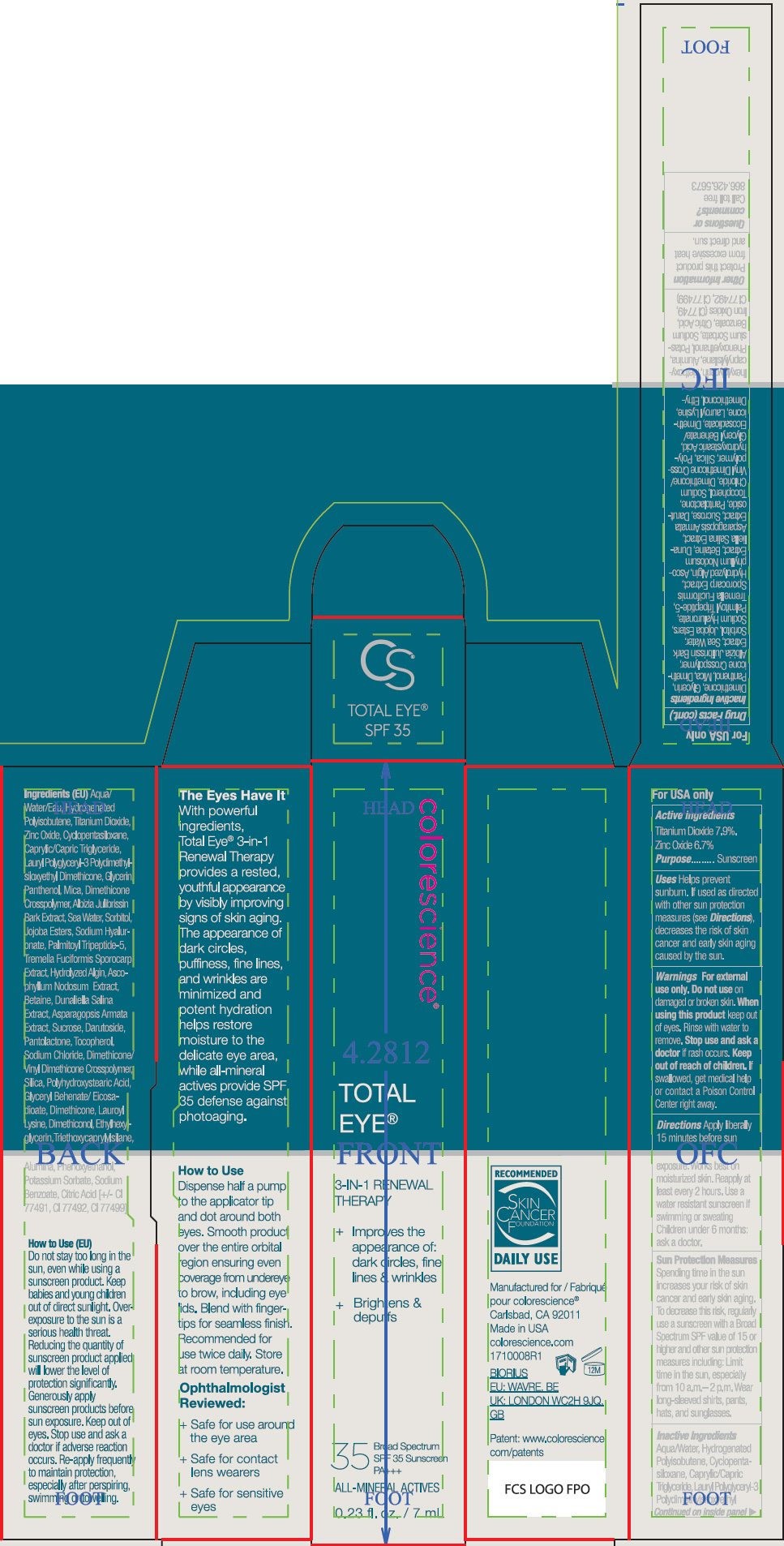
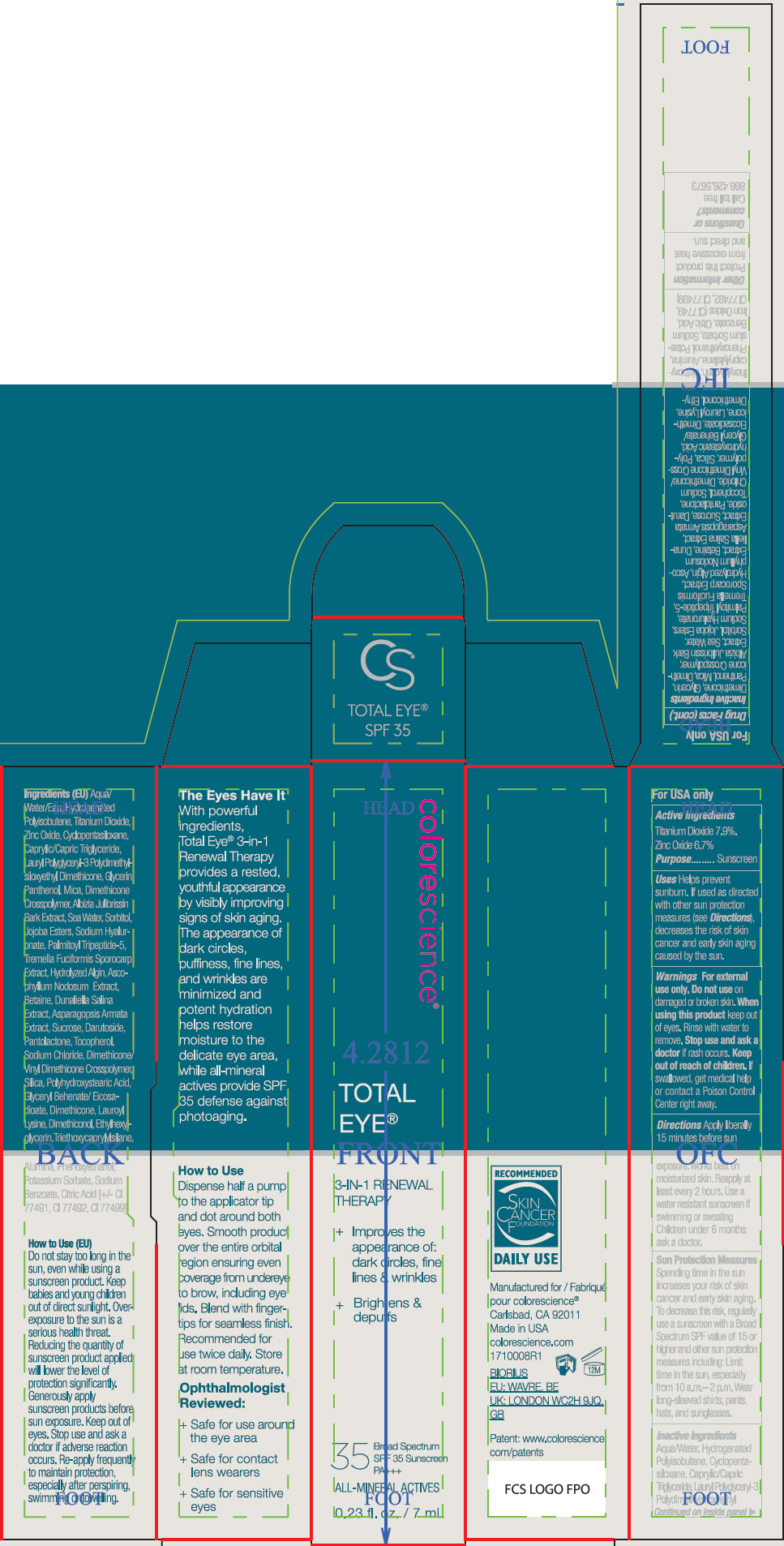
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure. Works best on moisturized skin.
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months: ask a doctor.
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.– 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
-
Inactive Ingredients
Aqua/Water, Hydrogenated Polyisobutene, Cyclopentasiloxane, Caprylic/Capric Triglyceride, Lauryl Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Glycerin, Panthenol, Mica, Dimethicone Crosspolymer, Albizia Julibrissin Bark Extract, Sea Water, Sorbitol, Jojoba Esters, Sodium Hyaluronate, Palmitoyl Tripeptide-5, Tremella Fuciformis Sporocarp Extract, Hydrolyzed Algin, Ascophyllum Nodosum Extract, Betaine, Dunaliella Salina Extract, Asparagopsis Armata Extract, Sucrose, Darutoside, Pantolactone, Tocopherol, Sodium Chloride, Dimethicone/Vinyl Dimethicone Crosspolymer, Silica, Polyhydroxystearic Acid, Glyceryl Behenate/Eicosadioate, Dimethicone, Lauroyl Lysine, Dimethiconol, Ethylhexylglycerin, Triethoxycaprylylsilane, Alumina, Phenoxyethanol, Potassium Sorbate, Sodium Benzoate, Citric Acid, Iron Oxides (CI 77491, CI 77492, CI 77499)
- Other Information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 7 mL Tube Carton
- PRINCIPAL DISPLAY PANEL - 7 mL Tube Carton - Fair
- PRINCIPAL DISPLAY PANEL - 7 mL Tube Carton - Tan
- PRINCIPAL DISPLAY PANEL - 7 mL Tube Carton - Deep
-
INGREDIENTS AND APPEARANCE
TOTAL EYE 3-IN-1 RENEWAL THERAPY SPF 35
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68078-024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 79 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 67 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Glycerin (UNII: PDC6A3C0OX) Panthenol (UNII: WV9CM0O67Z) Mica (UNII: V8A1AW0880) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) Sorbitol (UNII: 506T60A25R) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Palmitoyl Tripeptide-5 (UNII: 2A3916MQHO) ASCOPHYLLUM NODOSUM (UNII: 168S4EO8YJ) Betaine (UNII: 3SCV180C9W) DUNALIELLA SALINA (UNII: F4O1DKI9A6) ASPARAGOPSIS ARMATA (UNII: 2936KN6I1G) Sucrose (UNII: C151H8M554) Darutoside (UNII: EG8ODI0780) PANTOLACTONE, (+/-)- (UNII: IID733HD2M) Tocopherol (UNII: R0ZB2556P8) Sodium Chloride (UNII: 451W47IQ8X) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) Glyceryl Behenate/Eicosadioate (UNII: 73CJJ317SR) Dimethicone (UNII: 92RU3N3Y1O) Lauroyl Lysine (UNII: 113171Q70B) Ethylhexylglycerin (UNII: 147D247K3P) Triethoxycaprylylsilane (UNII: LDC331P08E) ALUMINUM OXIDE (UNII: LMI26O6933) Phenoxyethanol (UNII: HIE492ZZ3T) Potassium Sorbate (UNII: 1VPU26JZZ4) Sodium Benzoate (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68078-024-01 1 in 1 CARTON 01/19/2018 1 7 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:68078-024-02 0.5 mL in 1 PACKET; Type 0: Not a Combination Product 01/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 01/19/2018 TOTAL EYE 3-IN-1 RENEWAL THERAPY SPF 35 (FAIR)
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68078-046 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 79 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 67 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Glycerin (UNII: PDC6A3C0OX) Panthenol (UNII: WV9CM0O67Z) Mica (UNII: V8A1AW0880) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) Sorbitol (UNII: 506T60A25R) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Palmitoyl Tripeptide-5 (UNII: 2A3916MQHO) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) ASCOPHYLLUM NODOSUM (UNII: 168S4EO8YJ) Betaine (UNII: 3SCV180C9W) DUNALIELLA SALINA (UNII: F4O1DKI9A6) ASPARAGOPSIS ARMATA (UNII: 2936KN6I1G) Sucrose (UNII: C151H8M554) Darutoside (UNII: EG8ODI0780) PANTOLACTONE, (+/-)- (UNII: IID733HD2M) Tocopherol (UNII: R0ZB2556P8) Sodium Chloride (UNII: 451W47IQ8X) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) Glyceryl Behenate/Eicosadioate (UNII: 73CJJ317SR) Dimethicone (UNII: 92RU3N3Y1O) Lauroyl Lysine (UNII: 113171Q70B) Ethylhexylglycerin (UNII: 147D247K3P) Triethoxycaprylylsilane (UNII: LDC331P08E) ALUMINUM OXIDE (UNII: LMI26O6933) Phenoxyethanol (UNII: HIE492ZZ3T) Potassium Sorbate (UNII: 1VPU26JZZ4) Sodium Benzoate (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68078-046-01 1 in 1 CARTON 01/19/2018 1 7 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 01/19/2018 TOTAL EYE 3-IN-1 RENEWAL THERAPY SPF 35 (TAN)
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68078-047 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 79 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 67 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Glycerin (UNII: PDC6A3C0OX) Panthenol (UNII: WV9CM0O67Z) Mica (UNII: V8A1AW0880) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) Sorbitol (UNII: 506T60A25R) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Palmitoyl Tripeptide-5 (UNII: 2A3916MQHO) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) ASCOPHYLLUM NODOSUM (UNII: 168S4EO8YJ) Betaine (UNII: 3SCV180C9W) DUNALIELLA SALINA (UNII: F4O1DKI9A6) ASPARAGOPSIS ARMATA (UNII: 2936KN6I1G) Sucrose (UNII: C151H8M554) Darutoside (UNII: EG8ODI0780) PANTOLACTONE, (+/-)- (UNII: IID733HD2M) Tocopherol (UNII: R0ZB2556P8) Sodium Chloride (UNII: 451W47IQ8X) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) Glyceryl Behenate/Eicosadioate (UNII: 73CJJ317SR) Dimethicone (UNII: 92RU3N3Y1O) Lauroyl Lysine (UNII: 113171Q70B) Ethylhexylglycerin (UNII: 147D247K3P) Triethoxycaprylylsilane (UNII: LDC331P08E) ALUMINUM OXIDE (UNII: LMI26O6933) Phenoxyethanol (UNII: HIE492ZZ3T) Potassium Sorbate (UNII: 1VPU26JZZ4) Sodium Benzoate (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68078-047-01 1 in 1 CARTON 01/19/2018 1 7 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 01/19/2018 TOTAL EYE 3-IN-1 RENEWAL THERAPY SPF 35 (DEEP)
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68078-048 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 79 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 67 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Glycerin (UNII: PDC6A3C0OX) Panthenol (UNII: WV9CM0O67Z) Mica (UNII: V8A1AW0880) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) Sorbitol (UNII: 506T60A25R) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Palmitoyl Tripeptide-5 (UNII: 2A3916MQHO) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) ASCOPHYLLUM NODOSUM (UNII: 168S4EO8YJ) Betaine (UNII: 3SCV180C9W) DUNALIELLA SALINA (UNII: F4O1DKI9A6) ASPARAGOPSIS ARMATA (UNII: 2936KN6I1G) Sucrose (UNII: C151H8M554) Darutoside (UNII: EG8ODI0780) PANTOLACTONE, (+/-)- (UNII: IID733HD2M) Tocopherol (UNII: R0ZB2556P8) Sodium Chloride (UNII: 451W47IQ8X) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) Glyceryl Behenate/Eicosadioate (UNII: 73CJJ317SR) Dimethicone (UNII: 92RU3N3Y1O) Lauroyl Lysine (UNII: 113171Q70B) Ethylhexylglycerin (UNII: 147D247K3P) Triethoxycaprylylsilane (UNII: LDC331P08E) ALUMINUM OXIDE (UNII: LMI26O6933) Phenoxyethanol (UNII: HIE492ZZ3T) Potassium Sorbate (UNII: 1VPU26JZZ4) Sodium Benzoate (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68078-048-01 1 in 1 CARTON 01/19/2018 1 7 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 01/19/2018 Labeler - Colorescience (128731929)