Label: DELSYM- dextromethorphan polistirex syrup
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-517-30, 21695-518-30 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 63824-171, 63824-172
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 12, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
Warnings
Do not use
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI
drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
• chronic cough that lasts as occurs with smoking, asthma or emphysema
• cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if you are
When using this product
Stop use and ask a doctor if
Stop use and ask a doctor if cough lasts more than 7 days, cough comes back, or occurs with fever, rash or headache that lasts. These could be signs of a serious condition.
If pregnant or breast-feeding
Keep out of reach of children
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
-
Directions
Directions
• shake bottle well before use
• measure only with dosing cup provided. Do not use dosing cup with other products.
• dose as follows or as directed by a doctor
adults and children 12 years of age and over
10 mL every 12 hours,
not to exceed 20 mL in 24 hours
children 6 to under 12 years of age
5 mL every 12 hours,
not to exceed 10 mL in 24 hours
children 4 to under 6 years of age
2.5 mL every 12 hours,
not to exceed 5 mL in 24 hours
children under 4 years of age do not use
Other information
• each 5 mL contains: sodium 7 mg
• store at 20°-25°C (68°-77°F)
• dosing cup provided
Drug Facts
Distributed by: Reckitt Benckiser Inc., Parsippany, NJ 07054-0224 ©RBI 2009
- Inactive ingredients
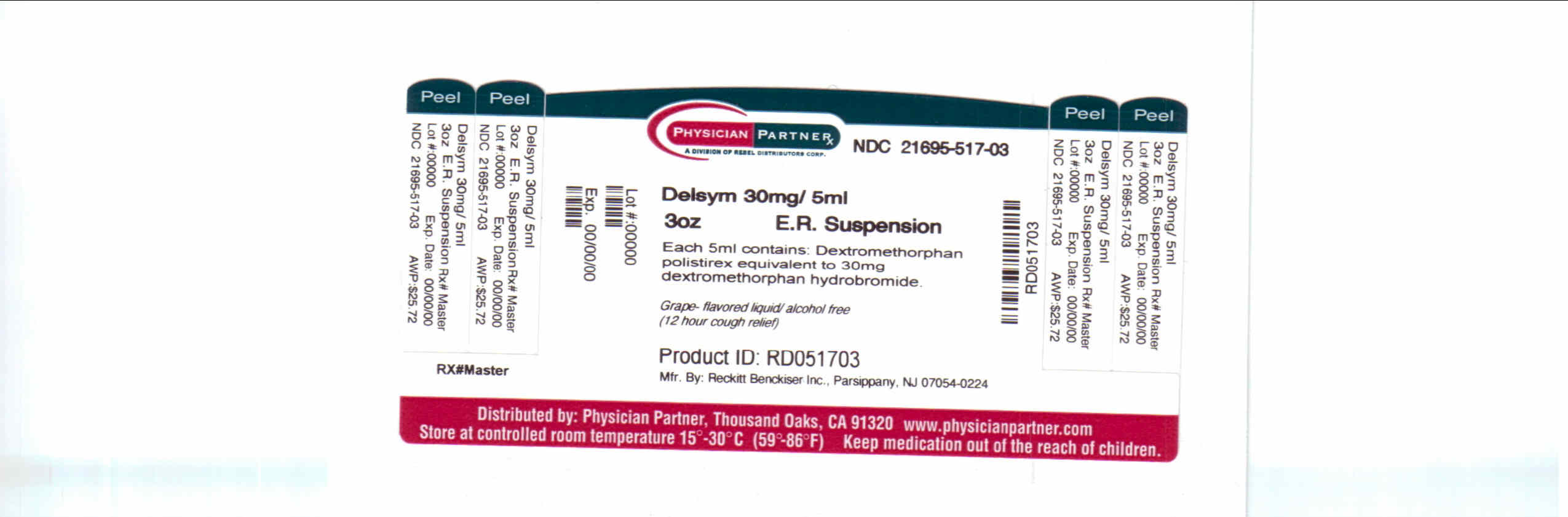
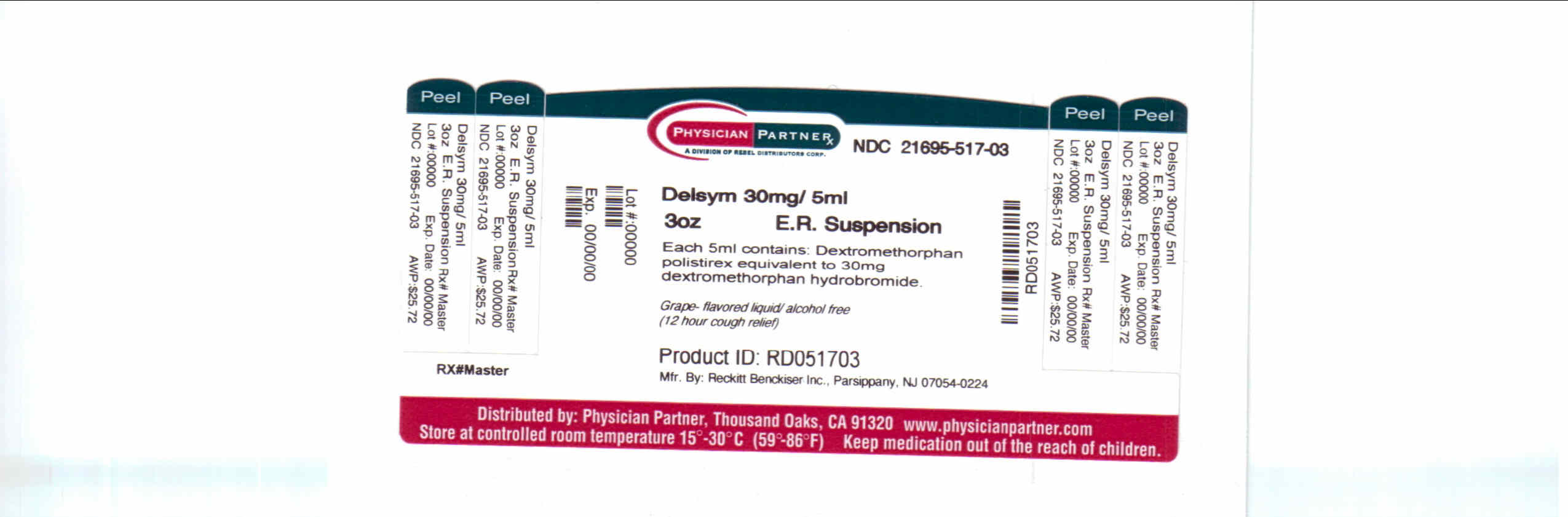
- Package/Label Principal Display Panel
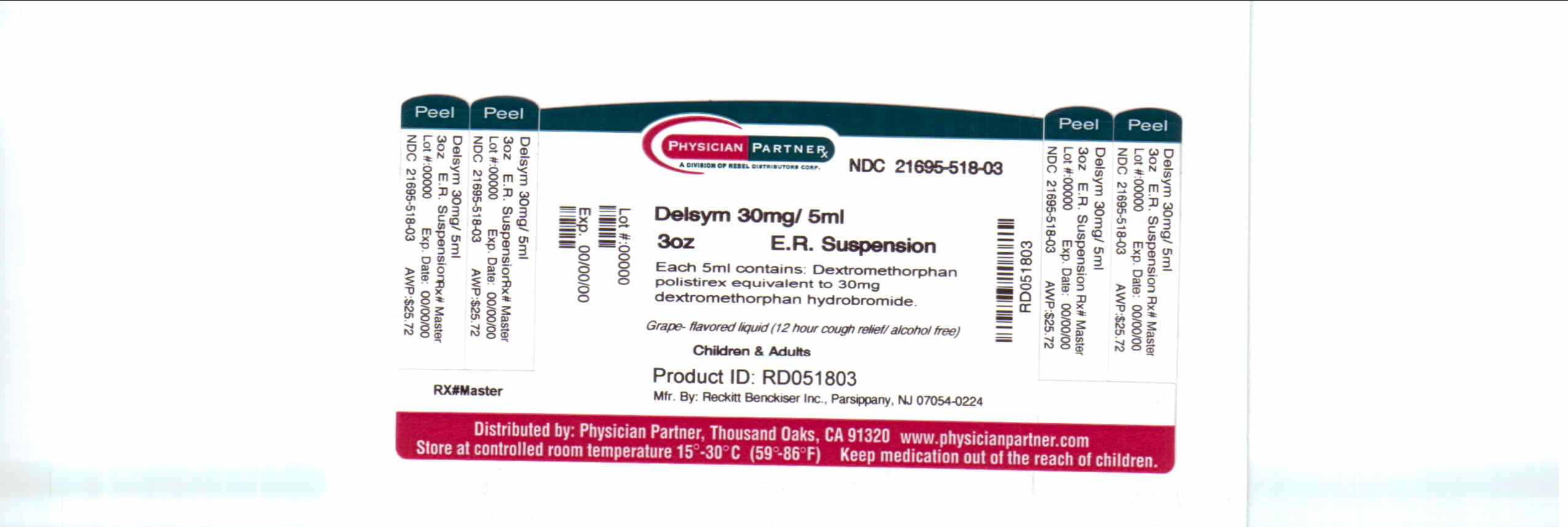
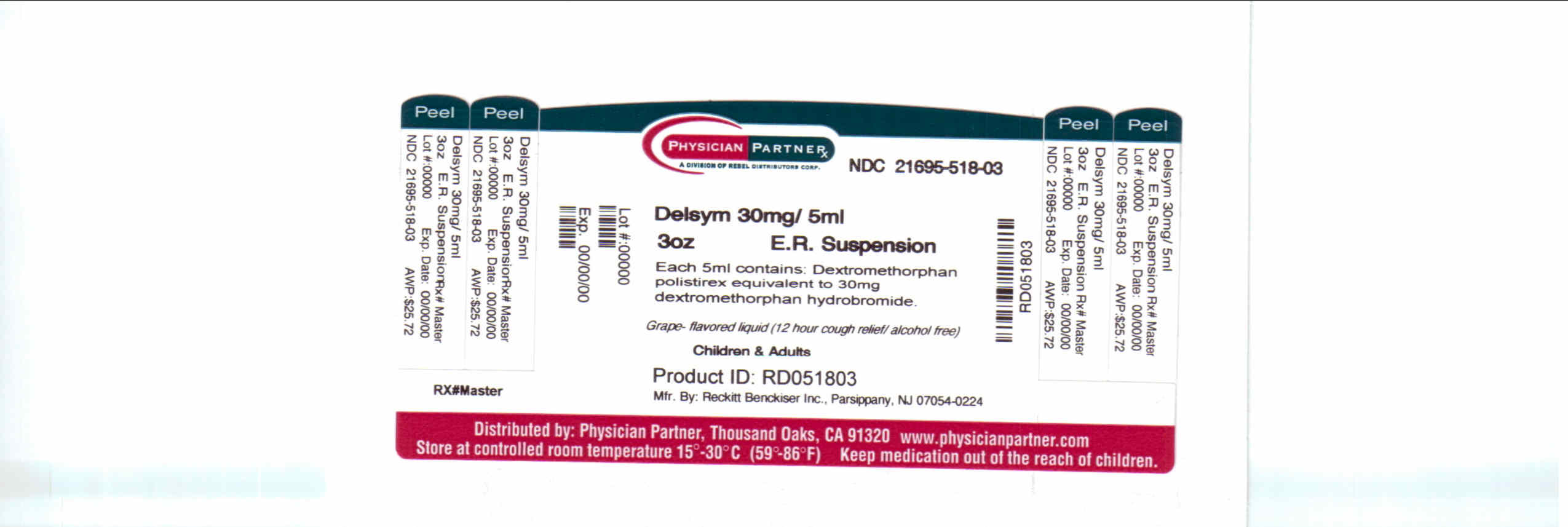
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DELSYM
dextromethorphan polistirex syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21695-517(NDC:63824-171) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN (UNII: 7355X3ROTS) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) D&C RED NO. 33 (UNII: 9DBA0SBB0L) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SUCROSE (UNII: C151H8M554) TRAGACANTH (UNII: 2944357O2O) CORN OIL (UNII: 8470G57WFM) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-517-30 90 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018658 09/04/2009 DELSYM
dextromethorphan polistirex syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21695-518(NDC:63824-172) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN (UNII: 7355X3ROTS) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) D&C RED NO. 33 (UNII: 9DBA0SBB0L) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SUCROSE (UNII: C151H8M554) TRAGACANTH (UNII: 2944357O2O) CORN OIL (UNII: 8470G57WFM) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-518-30 90 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018658 09/04/2009 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK