Label: CARADERMA- benzoyl peroxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 51130-333-12 - Packager: A Refreshing Discovery, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 4, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
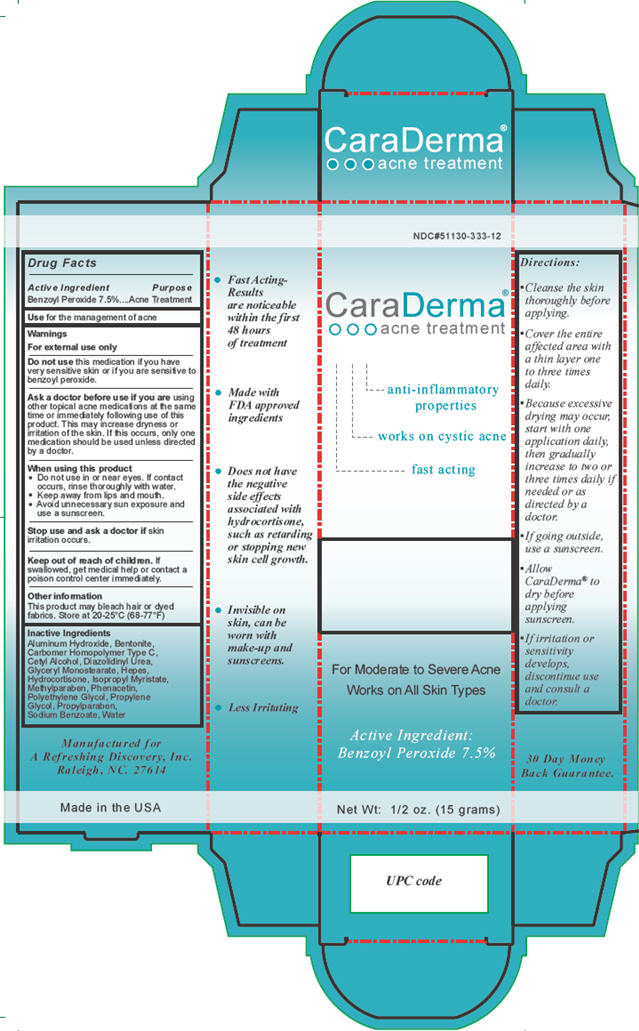
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Do not use this medication if you have very sensitive skin or if you are sensitive to benzoyl peroxide.
Ask a doctor before use if you are using other topical acne medications at the same time or immediately following use of this product. This may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
When using this product
- Do not use in or near eyes. If contact occurs, rinse thoroughly with water.
- Keep away from lips and mouth.
- Avoid unnecessary sun exposure and use a sunscreen.
Stop use and ask a doctor if skin irritation occurs.
- Inactive ingredients
-
Directions
- Cleanse the skin thoroughly before applying.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If going outside, use a sunscreen.
- Allow CaraDerma® to dry before applying sunscreen.
- If irritation or sensitivity develops, discontinue use and consult a doctor.
-
Package/Label Principal Display Panel
CaraDerma®
OOO acne treatment
anti-inflammatory properties
works on cystic acne
fast acting
For Moderate to Severe Acne
Works on All Skin Types
Active Ingredient:
Benzoyl Peroxide 7.5%
Net Wt: 1/2 oz. (15 grams)
Manufactured for
A Refreshing Discovery, Inc.
Raleigh, NC 27614
Made in the USA
Carton Label
-
INGREDIENTS AND APPEARANCE
CARADERMA
benzoyl peroxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51130-333 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 1.125 g in 15 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENTONITE (UNII: A3N5ZCN45C) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) CETYL ALCOHOL (UNII: 936JST6JCN) HYDROCORTISONE (UNII: WI4X0X7BPJ) PHENACETIN (UNII: ER0CTH01H9) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM BENZOATE (UNII: OJ245FE5EU) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) HYDROXYETHYLPIPERAZINE ETHANE SULFONIC ACID (UNII: RWW266YE9I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51130-333-12 1 in 1 CARTON 1 15 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333D 09/02/2010 Labeler - A Refreshing Discovery, Inc. (604440243) Establishment Name Address ID/FEI Business Operations Advanced Skin Technologies Inc. 176084556 MANUFACTURE