Label: ACETAMINOPHEN PM EXTRA STRENGTH- acetaminophen, diphenhydramine hcl tablet, coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 68391-161-05 - Packager: BJWC (Berkley & Jensen / BJ's)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 31, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
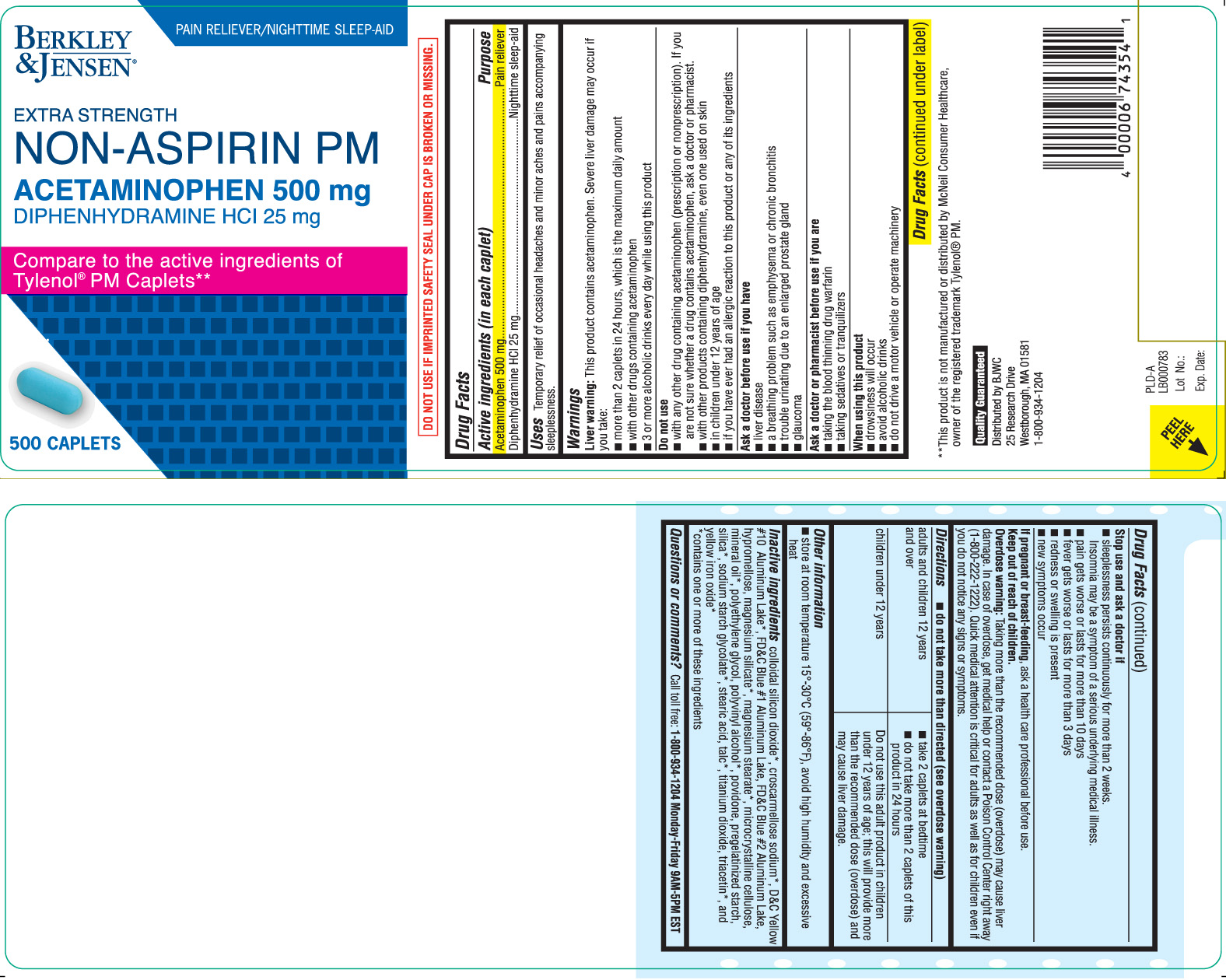
- Active ingredients (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 2 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with other products containing diphenhydramine, even one used on skin
- in children under 12 years of age
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
- pain gets worse or lasts for more than 10 days
- fever gets worse or lasts for more than 3 days
- redness or swelling is present
- new symptoms occur
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
-
Directions
-
do not take more than directed (see overdose warning)
adults and children 12 years and over - take 2 caplets at bedtime
- do not take more than 2 caplets of this product in 24 hours
children under 12 years Do not use this adult product in children under 12 years of age, this will provide more than the recommended dose (overdose) and may cause liver damage.
-
do not take more than directed (see overdose warning)
- Other information
-
Inactive ingredients
colloidal silicon dioxide*, croscarmellose sodium*, D&C Yellow #10 Aluminum Lake*, FD&C Blue #1 Aluminum Lake, FD&C Blue #2 Aluminum Lake, hypromellose, magnesium silicate*, magnesium stearate*, microcrystalline cellulose, mineral oil*, polyethylene glycol, polyvinyl alcohol*, povidone, pregelatinized starch, silica*, sodium starch glycolate*, stearic acid, talc*, titanium dioxide, triacetin*, and yellow iron oxide*
*contains one or more of these ingredients - Questions or comments?
-
Principal Display Panel
PAIN RELIEVER/NIGHTTIME SLEEP-AID
EXTRA STRENGTH
NON-ASPIRIN PM
ACETAMINOPHEN 500 mg
DIPHENHYDRAMINE HCl 25 mg
Compare to the active ingredients of Tylenol® PM Caplets**
CAPLETS
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
**This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Tylenol® PM.
Distributed by BJWC
25 Research Drive
Westborough, MA 01581
1-800-934-1204
- Package Label
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN PM EXTRA STRENGTH
acetaminophen, diphenhydramine hcl tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68391-161 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL (UNII: 532B59J990) POVIDONES (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) MAGNESIUM SILICATE (UNII: 9B9691B2N9) ALUMINUM OXIDE (UNII: LMI26O6933) MINERAL OIL (UNII: T5L8T28FGP) STARCH, CORN (UNII: O8232NY3SJ) TRIACETIN (UNII: XHX3C3X673) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color BLUE Score no score Shape CAPSULE Size 7mm Flavor Imprint Code S525;CPC752 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68391-161-05 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 01/15/2013 Labeler - BJWC (Berkley & Jensen / BJ's) (159082692) Registrant - P and L Development of New York Corporation (800014821)