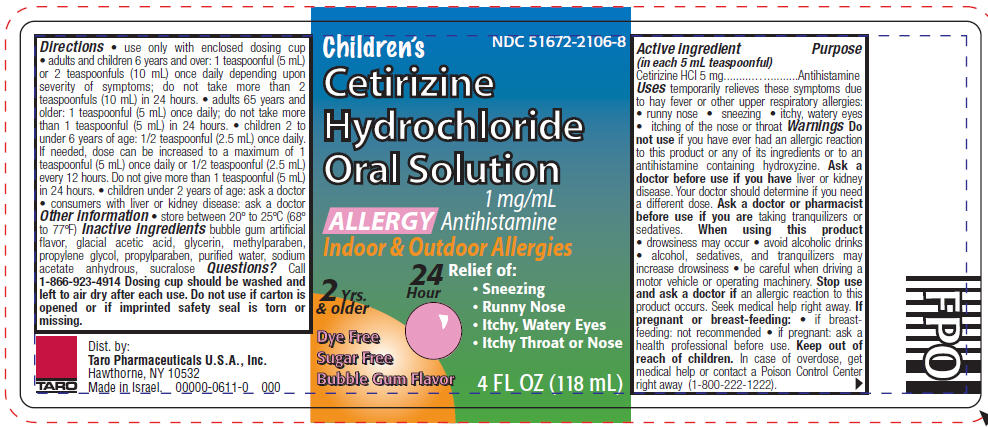
Label: CETIRIZINE HYDROCHLORIDE solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 52549-2106-1, 52549-2106-8 - Packager: Taro Pharmaceutical Industries, Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 11, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 5 mL teaspoonful)
- Purpose
- Uses
-
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery.
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
- use only with enclosed dosing cup
- adults and children 6 years and over: 1 teaspoonful (5 mL) or 2 teaspoonfuls (10 mL) once daily depending upon severity of symptoms; do not take more than 2 teaspoonfuls (10 mL) in 24 hours.
- adults 65 years and older: 1 teaspoonful (5 mL) once daily; do not take more than 1 teaspoonful (5 mL) in 24 hours.
- children 2 to under 6 years of age: 1/2 teaspoonful (2.5 mL) once daily. If needed, dose can be increased to a maximum of 1 teaspoonful (5 mL) once daily or 1/2 teaspoonful (2.5 mL) every 12 hours. Do not give more than 1 teaspoonful (5 mL) in 24 hours.
- children under 2 years of age: ask a doctor
- consumers with liver or kidney disease: ask a doctor
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52549-2106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetirizine Hydrochloride (UNII: 64O047KTOA) (Cetirizine - UNII:YO7261ME24) Cetirizine Hydrochloride 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength acetic acid (UNII: Q40Q9N063P) glycerin (UNII: PDC6A3C0OX) methylparaben (UNII: A2I8C7HI9T) propylene glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) sodium acetate anhydrous (UNII: NVG71ZZ7P0) sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color YELLOW (colorless to slightly yellow) Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52549-2106-8 1 in 1 CARTON 1 118 mL in 1 BOTTLE 2 NDC:52549-2106-1 1 in 1 CARTON 2 237 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201546 05/20/2011 Labeler - Taro Pharmaceutical Industries, Ltd. (600072078) Establishment Name Address ID/FEI Business Operations Taro Pharmaceutical Industries, Ltd. 600072078 MANUFACTURE