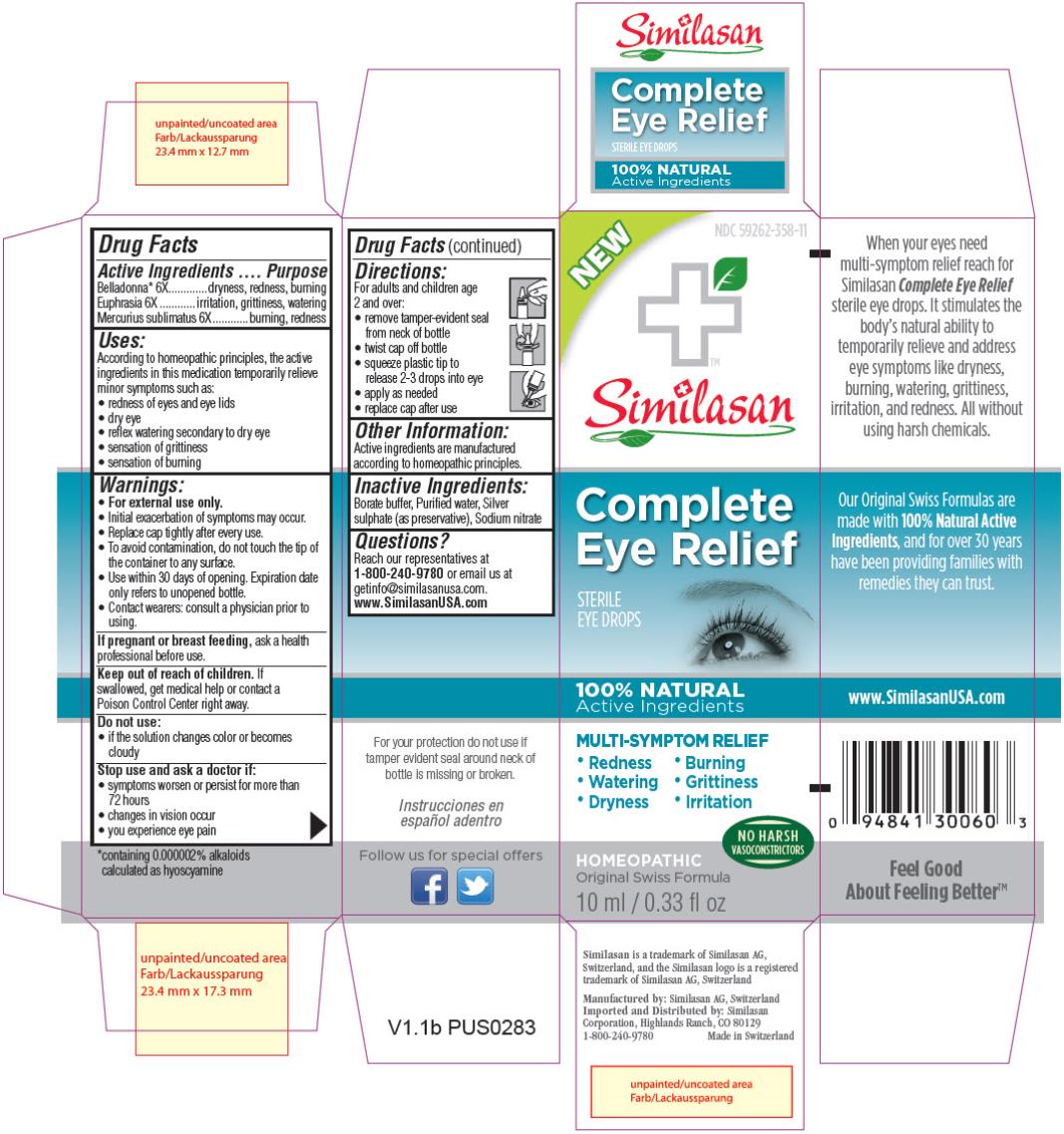
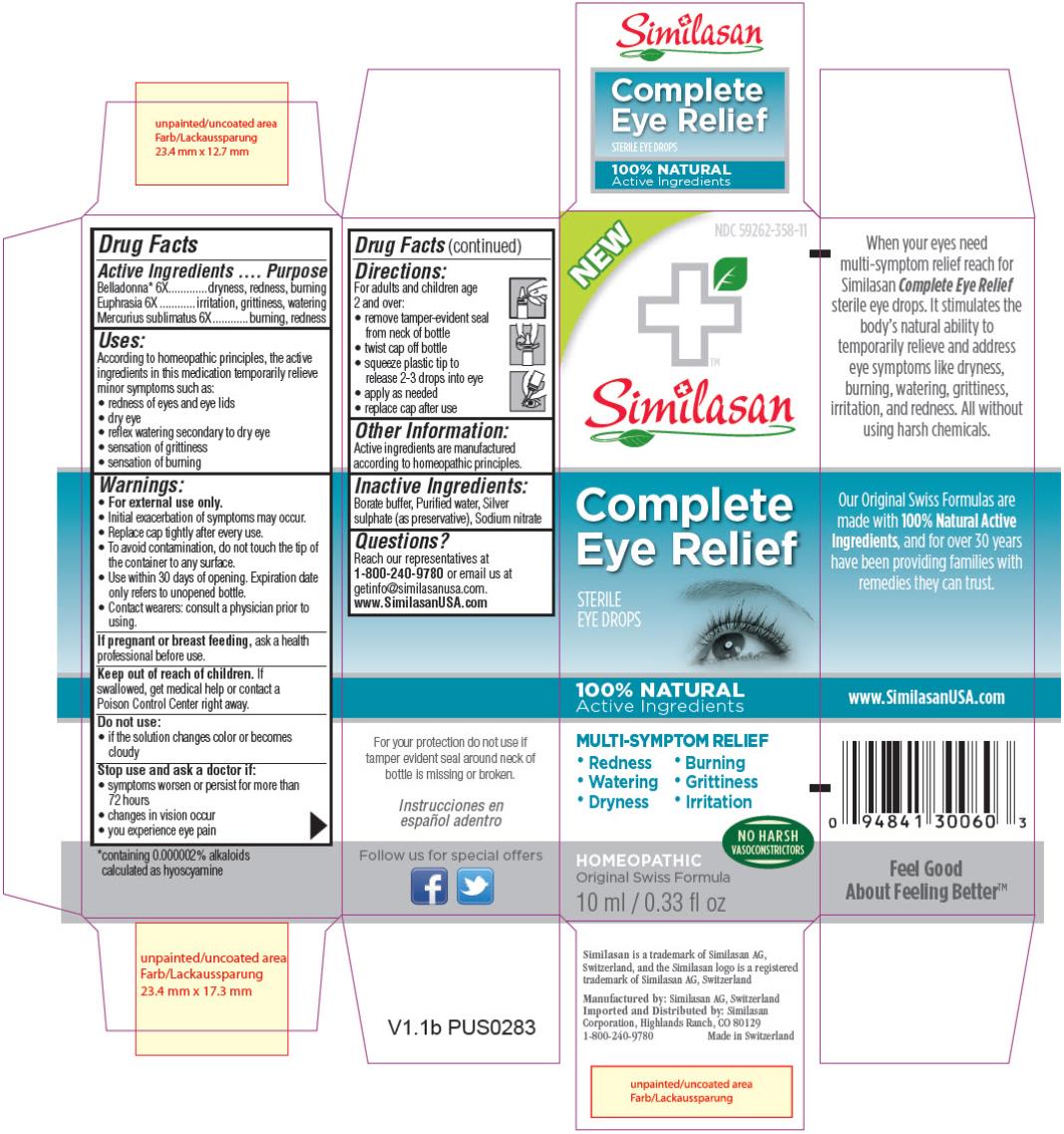
Label: COMPLETE EYE RELIEF- atropa belladonna, euphrasia stricta and mercuric chloride solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 59262-358-11 - Packager: Similasan Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 9, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Uses:
-
Warnings:
- For external use only.
- Initial exacerbation of symptoms may occur.
- Replace cap tightly after every use.
- To avoid contamination, do not touch the tip of the container to any surface.
- Use within 30 days of opening. Expiration date only refers to unopened bottle.
- Contact wearers: consult a physician prior to using.
- For external use only.
- If pregnant or breast feeding,
- Keep out of reach of children.
- Do not use:
- Stop use and ask a doctor if:
- Directions:
- Other Information:
- Inactive Ingredients:
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COMPLETE EYE RELIEF
atropa belladonna, euphrasia stricta and mercuric chloride solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59262-358 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 6 [hp_X] in 10 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 10 mL MERCURIC CHLORIDE (UNII: 53GH7MZT1R) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC CHLORIDE 6 [hp_X] in 10 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) WATER (UNII: 059QF0KO0R) SILVER SULFATE (UNII: 8QG6HV4ZPO) SODIUM NITRATE (UNII: 8M4L3H2ZVZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59262-358-11 1 in 1 BOX 1 10 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 02/11/2014 Labeler - Similasan Corporation (111566530) Establishment Name Address ID/FEI Business Operations Similasan AG 481545754 LABEL(59262-358) , MANUFACTURE(59262-358) , PACK(59262-358)