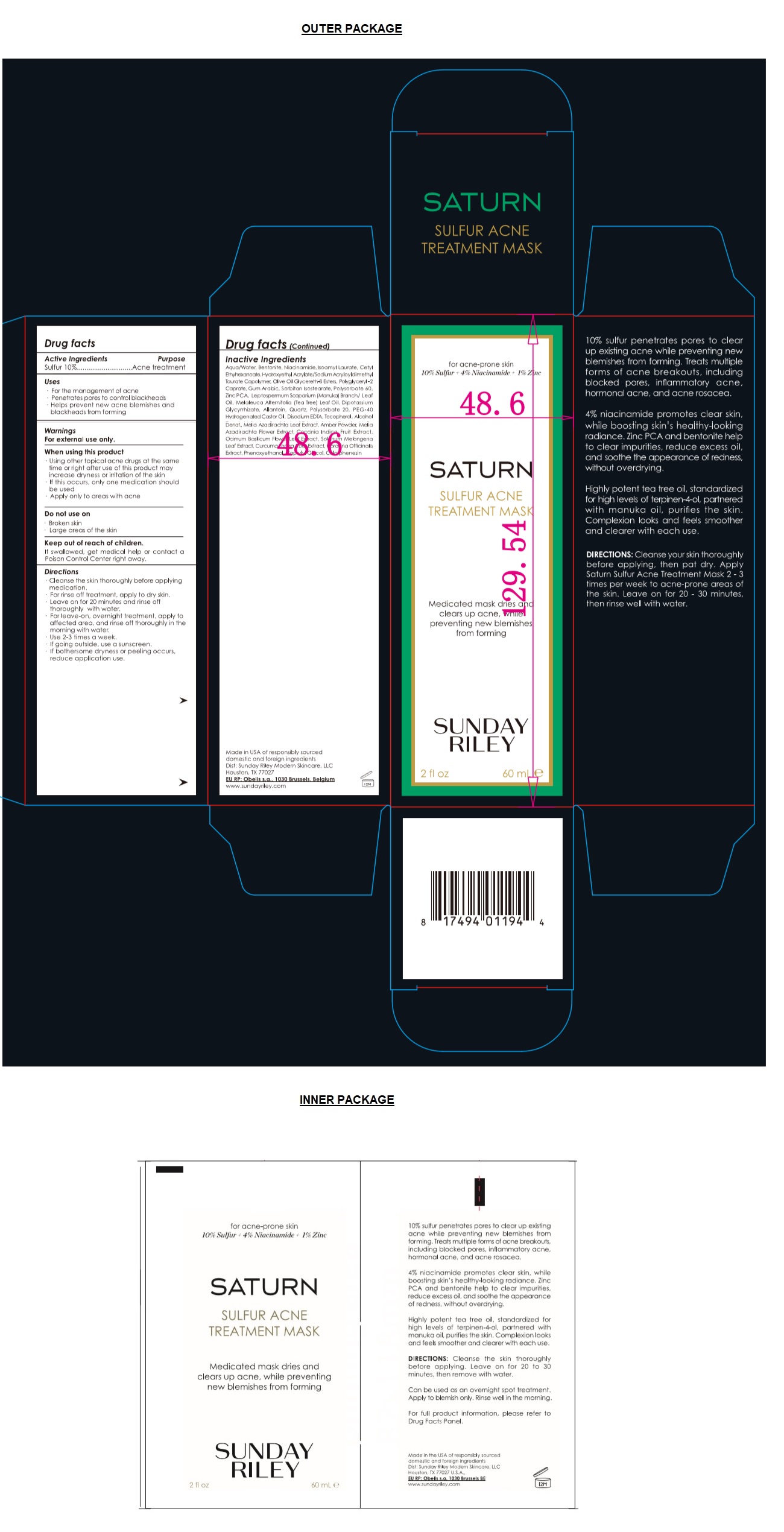
Label: SUNDAY RILEY SULFUR ACNE TREATMENT MASK- sulfur cream
- NDC Code(s): 73001-340-60
- Packager: Sunday Riley Modern Skincare, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 4, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug facts
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only.
When using this product
- Using other topical acne drugs at the same time or right after use of this product may increase dryness or irritation of the skin
- if this occurs, only one medication should be used
- Apply only to areas with acne
Do not use on
- Broken skin
- Large areas of the skin
-
Directions
- Cleanse the skin thoroughly before applying medication.
- For rinse off treatment, apply to dry skin.
- Leave on for 20 minutes and rinse off thoroughly with water.
- For leave-on, overnight treatment, apply to affected area, and rinse off thoroughly in the morning with water.
- Use 2-3 times a week.
- If going outside, use a sunscreen.
- If bothersome dryness or peeling occurs, reduce application use.
-
Inactive Ingredients
Aqua/Water, Bentonite, Niacinamide, Isoamyl Laurate, Cetyl Ethyhexanoate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Olive Oil Glycereth-8 Esters, Polyglyceryl-2 Caprate, Gum Arabic, Sorbitan Isostearate, Polysorbate 60, Zinc PCA, Leptospermum Scoparium (Manuca) Brach/Leaf Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Dipotassium Glycyrrhizate, Allantoin, Quartz, Plosorbate 20, PEG-40 Hydrogenated Castor Oil, Disodium EDTA, Tocopherol, Alcohol Denat., Melia Azadirachta Leaf Extract, Amber Powder, Melia Azadirachta Flower Extract, Coccinia Indica Fruit Extract, Ocimum Basilicum Flower/Leaf Extract, Solanum Melongena Leaf Extract, Curcuma Longa Root Extract, Corallina Officinalis Extract, Phenoxyethanol, Caprylyl Glycol, Chlorphenesin
-
SPL UNCLASSIFIED SECTION
for acne-prone skin
10% Sulfur + 4% Niacinamide + 1% Zinc
SATURN
Medicated mask dries and clears up acne, while preventing new blemishes from forming
10% sulfur penetrates pores to clear up existing acne while preventing new blemishes from forming. Treats multiple forms of acne breakouts, including blocked pores, inflammatory acne, hormonal acne and acne rosacea.
4% niacinamide promotes clear skin, while boosting skin's healthy-looking radiance. Zinc PCA and bentonite help to clear impurities, reduce excess oil, and soothe the appearance of redness, without overdrying.
Highly potent tea tree oil, standardized for high levels of terpinen-4-ol, partnered with manuka oil, purifies the skin, Complexion looks and feels smoother and clearer with each use.
Made in USA of responsibly sourced domestic and foreign ingredients
Dist: Sunday Riley Modern Skincare, LLC
Houston, TX 77027
EU RP: Obelis s.a., 1030 Brussels, Belgium
www.sundayriley.com
- Packaging
-
INGREDIENTS AND APPEARANCE
SUNDAY RILEY SULFUR ACNE TREATMENT MASK
sulfur creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73001-340 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 0.1 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENTONITE (UNII: A3N5ZCN45C) NIACINAMIDE (UNII: 25X51I8RD4) ISOAMYL LAURATE (UNII: M1SLX00M3M) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) OLIVE OIL GLYCERETH-8 ESTERS (UNII: 322K2STO13) POLYGLYCERYL-2 CAPRATE (UNII: JX7WXJ41DH) ACACIA (UNII: 5C5403N26O) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) POLYSORBATE 60 (UNII: CAL22UVI4M) ZINC PIDOLATE (UNII: C32PQ86DH4) MANUKA OIL (UNII: M6QU9ZUH2X) 4-TERPINEOL, (+/-)- (UNII: L65MV77ZG6) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) ALLANTOIN (UNII: 344S277G0Z) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) TOCOPHEROL (UNII: R0ZB2556P8) ALCOHOL (UNII: 3K9958V90M) AZADIRACHTA INDICA LEAF (UNII: HKY915780T) AMBER (UNII: 70J9Z0J26P) AZADIRACHTA INDICA FLOWER (UNII: 3TE8A92UPM) COCCINIA GRANDIS FRUIT (UNII: VLJ6WOT3K5) OCIMUM BASILICUM FLOWERING TOP (UNII: 7SAB275FP2) EGGPLANT (UNII: W5K7RAS4VK) TURMERIC (UNII: 856YO1Z64F) CORALLINA OFFICINALIS (UNII: 4004498D06) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73001-340-60 1 in 1 BOX 01/08/2017 1 60 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 01/08/2017 Labeler - Sunday Riley Modern Skincare, LLC (012302640)