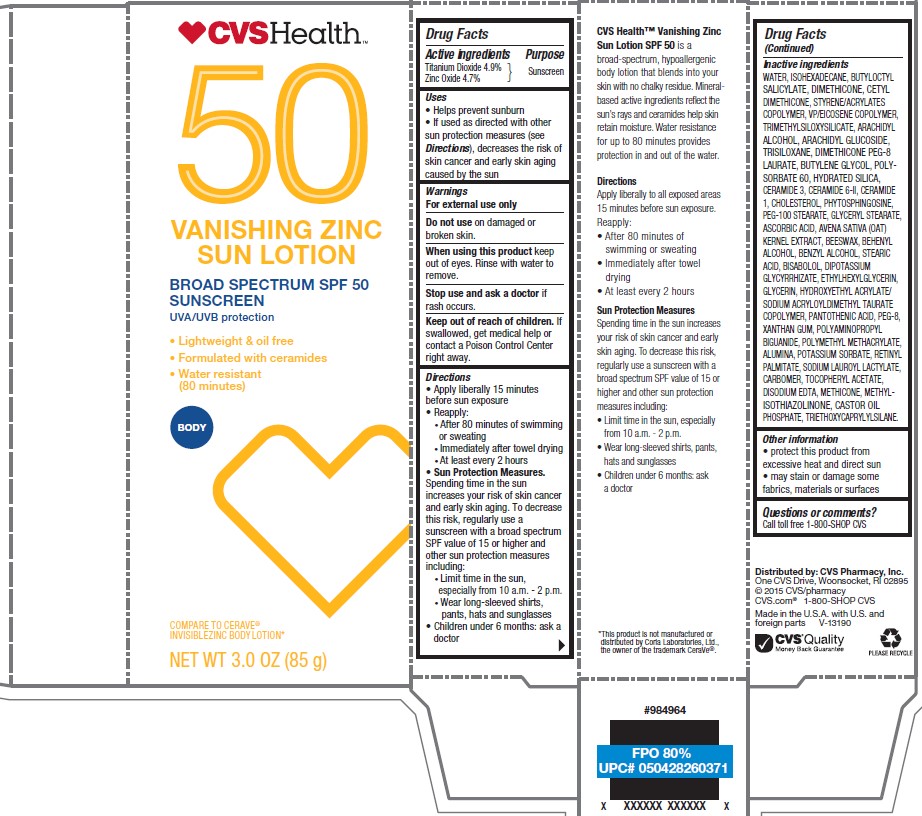
Label: CVS HEALTH SPF 50 VANISHING ZINC SUN- titanium dioxide, zinc oxide lotion
- NDC Code(s): 69842-142-03
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask doctor if
- Keep out of reach of children.
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
- Other information
-
Inactive ingredients
Water, Isohexadecane, Butyloctyl Salicylate, Dimethicone, Cetyl Dimethicone, Styrene/Acrylates Copolymer, VP/Eicosene Copolymer, Trimethylsiloxysilicate, Arachidyl Alcohol, Arachidyl Glucoside, Trisiloxane, Dimethicone PEG-8 Laurate, Butylene Glycol, Polysorbate 60, Hydrated Silica, Ceramide 3, Ceramide 6-II, Ceramide 1, Cholesterol, Phytosphingosine, PEG-100 Stearate, Glyceryl Stearate, Ascorbic Acid, Avena Sativa (Oat) Kernel Extract, Beeswax, Behenyl Alcohol, Benzyl Alcohol, Stearic Acid, Bisabolol, Dipotassium Glycyrrhizate, Ethylhexylglycerin, Glycerin, Hydroxyethyl Acrylate/sodium Acryloyldimethyl Taurate Copolymer, Pantothenic Acid, PEG-8, Xanthan Gum, Polyaminopropyl Biguanide, Polymethyl Methacrylate, Alumina, Potassium Sorbate, Retinyl Palmitate, Sodium Lauroyl Lactylate, Carbomer, Tocopheryl Acetate, Disodium EDTA, Methicone, Methylisothiazolinone, Castor Oil Phosphate, Triethoxycaprylylsilane.
- Label
-
INGREDIENTS AND APPEARANCE
CVS HEALTH SPF 50 VANISHING ZINC SUN
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-142 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 47 mg in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 49 mg in 1 g Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) CERAMIDE NG (UNII: C04977SRJ5) POLYAMINOPROPYL BIGUANIDE (UNII: DT9D8Z79ET) CERAMIDE 6 II (UNII: F1X8L2B00J) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) OAT (UNII: Z6J799EAJK) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ASCORBIC ACID (UNII: PQ6CK8PD0R) CERAMIDE 1 (UNII: 5THT33P7X7) ISOHEXADECANE (UNII: 918X1OUF1E) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 60 (UNII: CAL22UVI4M) DIMETHICONE PEG-8 LAURATE (UNII: 72MF9C2A18) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) WATER (UNII: 059QF0KO0R) YELLOW WAX (UNII: 2ZA36H0S2V) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PANTOTHENIC ACID (UNII: 19F5HK2737) STEARIC ACID (UNII: 4ELV7Z65AP) CASTOR OIL PHOSPHATE (UNII: SBR4NJI3UJ) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) XANTHAN GUM (UNII: TTV12P4NEE) PEG-100 STEARATE (UNII: YD01N1999R) DOCOSANOL (UNII: 9G1OE216XY) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LEVOMENOL (UNII: 24WE03BX2T) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) CHOLESTEROL (UNII: 97C5T2UQ7J) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) TRISILOXANE (UNII: 9G1ZW13R0G) CETYL DIMETHICONE 150 (UNII: 5L694Y0T22) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) METHICONE (20 CST) (UNII: 6777U11MKT) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-142-03 85 g in 1 BOTTLE; Type 0: Not a Combination Product 02/03/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/03/2017 Labeler - CVS Pharmacy (062312574)