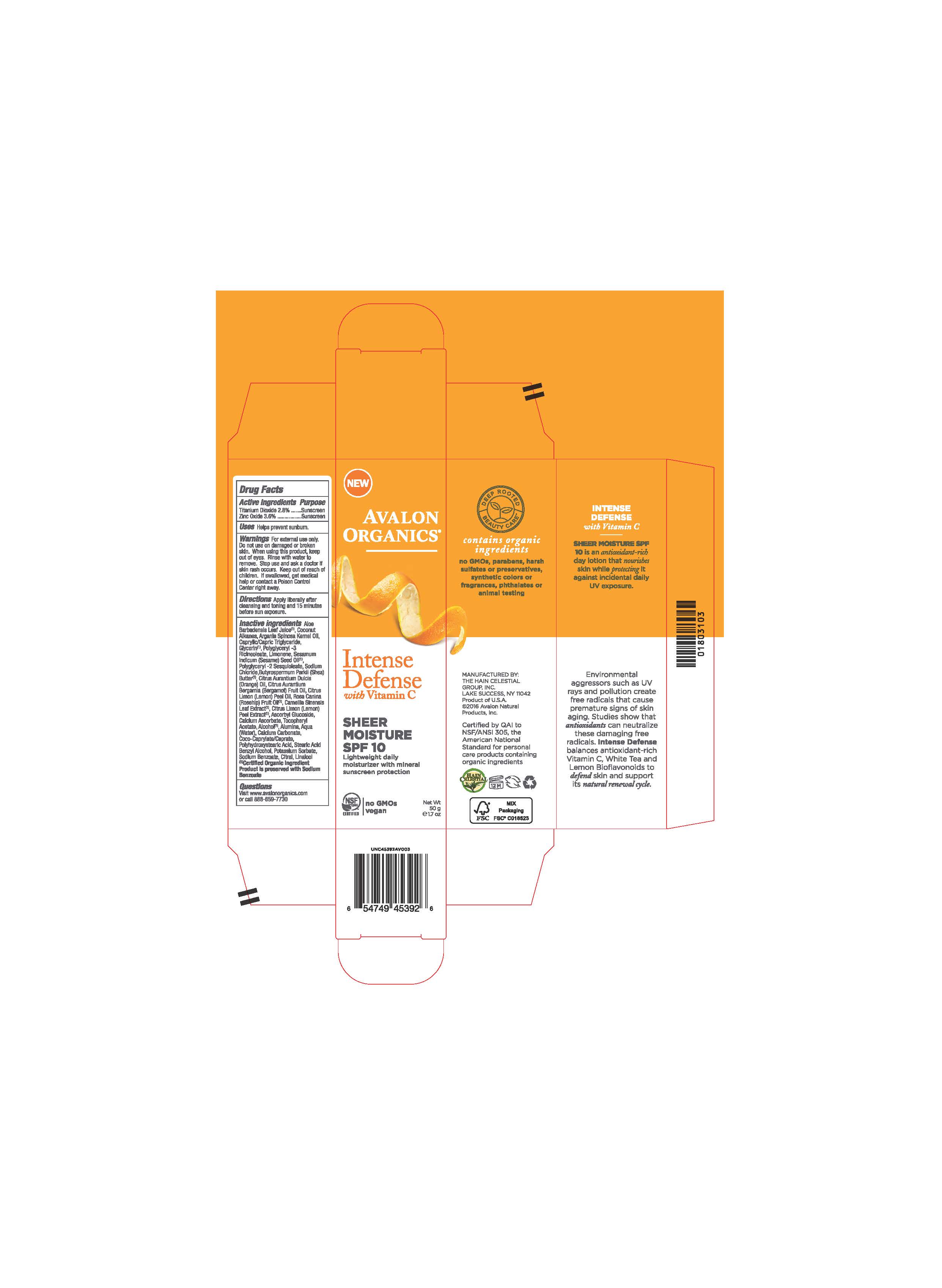
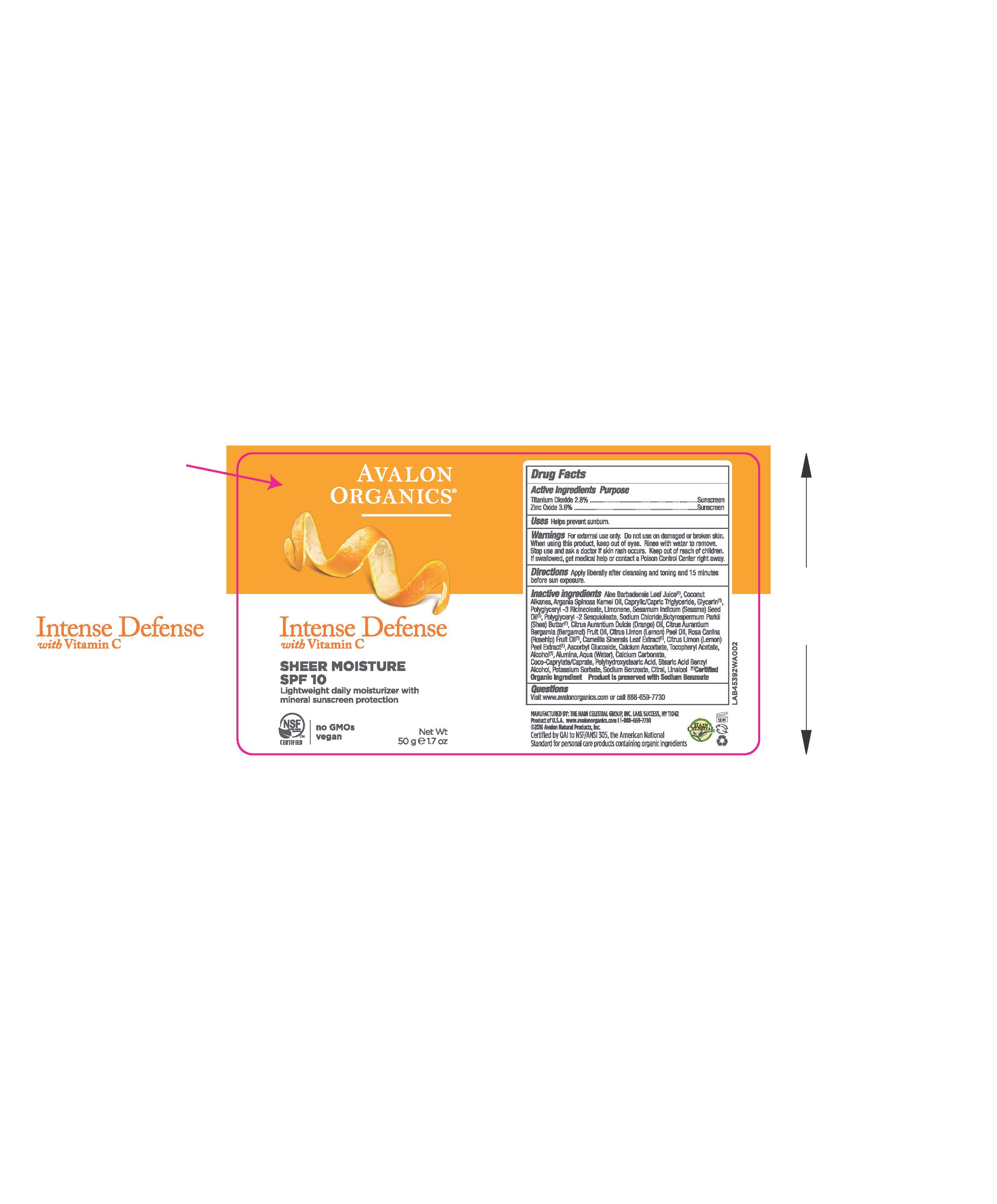
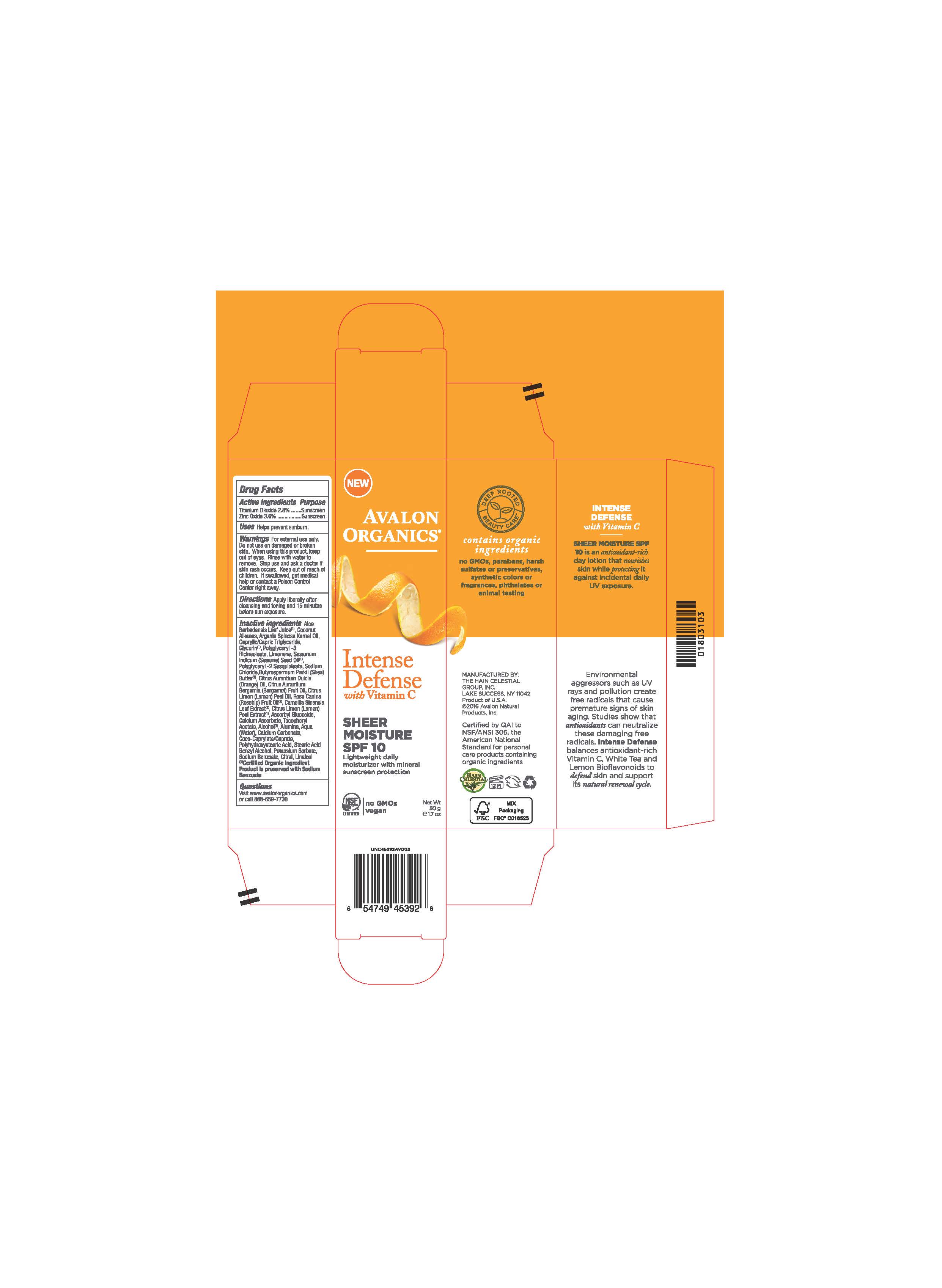
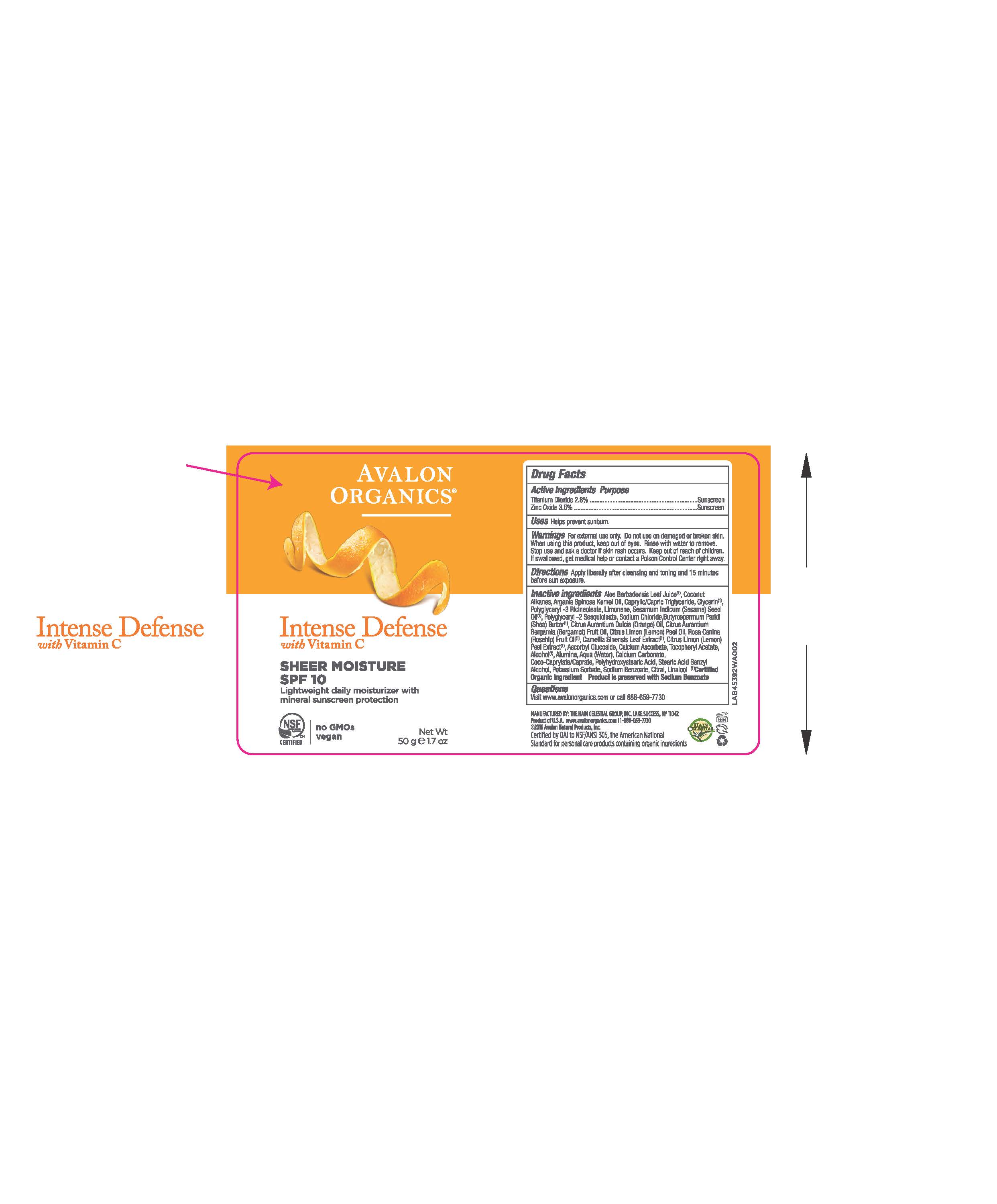
Label: AV453902 AVALON ORGANICS INTENSE DEFENSE SHEER MOISTURE SPF10- zinc oxide, titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 61995-1392-2 - Packager: The Hain Celestial Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 5, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Aloe Barbadensis Leaf Juice(1),Coconut Alkanes, Argania Spinosa Kernel Oil (1), Caprylic Capric Triglyceride, Glycerin, Polyglyceryl-3 Ricinoleate, Limonene, Sesamum Indicum (Sesame) Seed Oil, Polyglyceryl-2 Sesquioleate, Sodium Chloride, Butyrospermum Parkii (Shea) Butter, Citrus Aurantium Dulcis (Orange) Peel Oil, Citrus Aurantium Bergamia (Bergamot) Fruit Oil, Citrus Limon (Lemon) Peel Oil, Rosa Canina (Rosehip) Fruit Oil (1), Camellia Sinensis Leaf Extract (1), Citrus Limon (Lemon) Peel Extract (1), Polyhydroxystearic Acid,Tocopheryl Acetate, Ascorbyl Glucoside, Alumina, Water,Calcium Ascorbate, Calcium Carbonate, Coco-Caprylate/Caprate, Sodium Chloride, Stearic Acid, Alcohol (1), Benzyl Alcohol, Potassium Sorbate, Sodium Benzoate, Citral, Linalool.
(1) Certified Organicf Ingredient
- INDICATIONS & USAGE
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AV453902 AVALON ORGANICS INTENSE DEFENSE SHEER MOISTURE SPF10
zinc oxide, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61995-1392 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.8 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 3.6 g in 100 g Inactive Ingredients Ingredient Name Strength LEMON OIL (UNII: I9GRO824LL) ROSA CANINA FRUIT OIL (UNII: CR7307M3QZ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) LEMON (UNII: 24RS0A988O) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) CALCIUM CARBONATE (UNII: H0G9379FGK) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRAL (UNII: T7EU0O9VPP) LINALOOL, (+/-)- (UNII: D81QY6I88E) BERGAMOT OIL (UNII: 39W1PKE3JI) SODIUM BENZOATE (UNII: OJ245FE5EU) ORANGE OIL (UNII: AKN3KSD11B) CALCIUM ASCORBATE (UNII: 183E4W213W) ALCOHOL (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) ALOE VERA LEAF (UNII: ZY81Z83H0X) COCONUT ALKANES (UNII: 1E5KJY107T) GLYCERIN (UNII: PDC6A3C0OX) POLYGLYCERYL-3 RICINOLEATE (UNII: MZQ63P0N0W) WATER (UNII: 059QF0KO0R) SHEA BUTTER (UNII: K49155WL9Y) ALUMINUM OXIDE (UNII: LMI26O6933) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) SODIUM CHLORIDE (UNII: 451W47IQ8X) STEARIC ACID (UNII: 4ELV7Z65AP) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LIMONENE, (+)- (UNII: GFD7C86Q1W) SESAME OIL (UNII: QX10HYY4QV) POLYGLYCERYL-2 SESQUIISOSTEARATE (UNII: LA272Q68GQ) ARGAN OIL (UNII: 4V59G5UW9X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61995-1392-2 1 in 1 CARTON 10/03/2016 1 1 in 1 CARTON 1 50 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/03/2016 Labeler - The Hain Celestial Group, Inc. (117115556) Registrant - The Hain Celestial Group, Inc. (014334364) Establishment Name Address ID/FEI Business Operations The Hain Celestial Group, Inc. 081512382 manufacture(61995-1392)