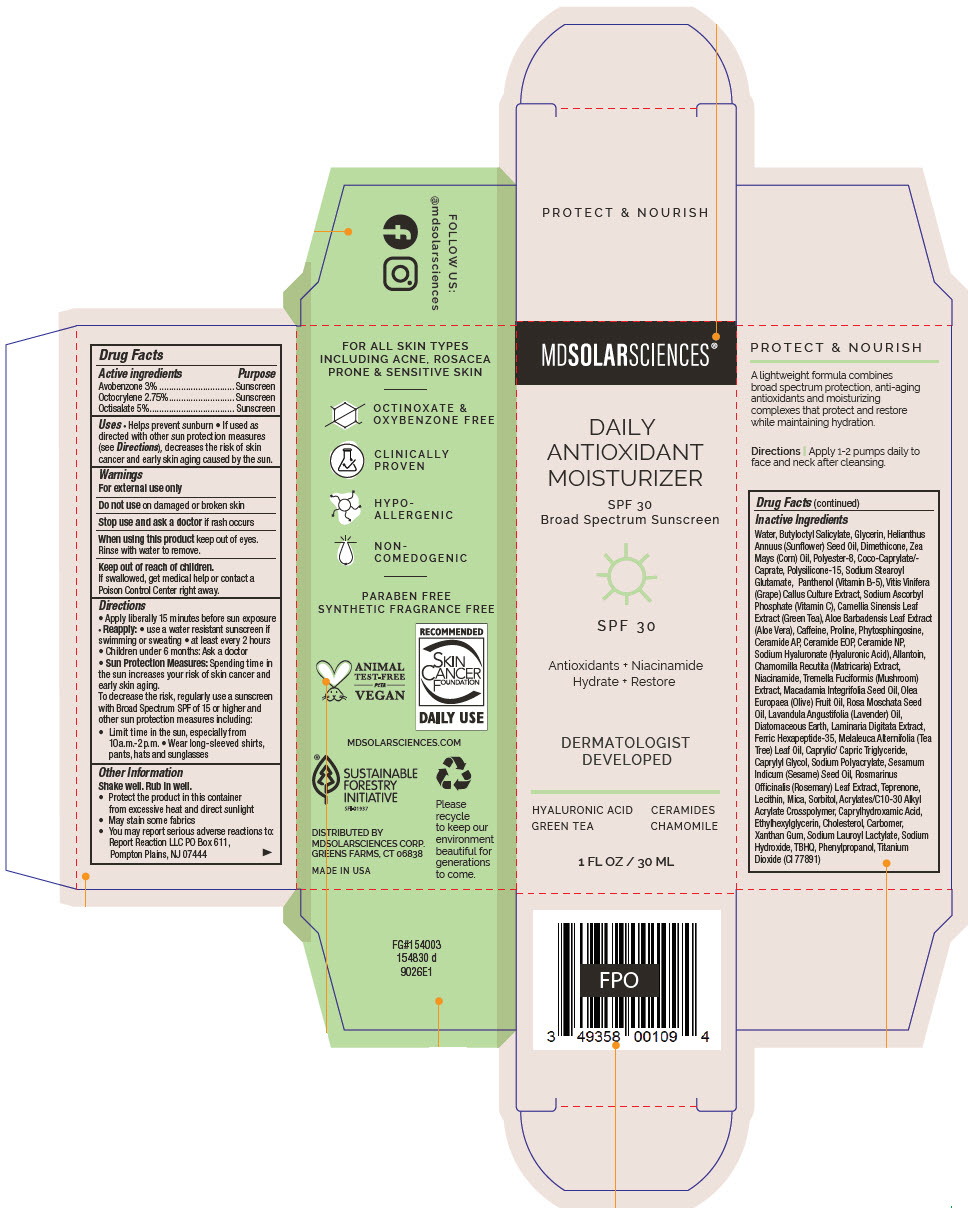
Label: DAILY ANTIOXIDANT MOISTURIZER SPF 30. SPF 30 BROAD SPECTRUM SUNSCREEN UVA-UVB- avobenzone, octocrylene, and octisalate lotion
- NDC Code(s): 49358-580-01
- Packager: MDSolarSciences
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
-
Reapply:
- use a water resistant sunscreen if swimming or sweating
- at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease the risk, regularly use a sunscreen with Broad Spectrum SPF of 15 or higher and other sun protection measures including:- Limit time in the sun, especially from 10a.m.-2p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other Information
-
Inactive Ingredients
Water, Butyloctyl Salicylate, Glycerin, Helianthus Annuus (Sunflower) Seed Oil, Dimethicone, Zea Mays (Corn) Oil, Polyester-8, Coco-Caprylate/-Caprate, Polysilicone-15, Sodium Stearoyl Glutamate, Panthenol (Vitamin B-5), Vitis Vinifera (Grape) Callus Culture Extract, Sodium Ascorbyl Phosphate (Vitamin C), Camellia Sinensis Leaf Extract (Green Tea), Aloe Barbadensis Leaf Extract (Aloe Vera), Caffeine, Proline, Phytosphingosine, Ceramide AP, Ceramide EOP, Ceramide NP, Sodium Hyaluronate (Hyaluronic Acid), Allantoin, Chamomilla Recutita (Matricaria) Extract, Niacinamide, Tremella Fuciformis (Mushroom) Extract, Macadamia Integrifolia Seed Oil, Olea Europaea (Olive) Fruit Oil, Rosa Moschata Seed Oil, Lavandula Angustifolia (Lavender) Oil, Diatomaceous Earth, Laminaria Digitata Extract, Ferric Hexapeptide-35, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Caprylic/ Capric Triglyceride, Caprylyl Glycol, Sodium Polyacrylate, Sesamum Indicum (Sesame) Seed Oil, Rosmarinus Officinalis (Rosemary) Leaf Extract, Teprenone, Lecithin, Mica, Sorbitol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylhydroxamic Acid, Ethylhexylglycerin, Cholesterol, Carbomer, Xanthan Gum, Sodium Lauroyl Lactylate, Sodium Hydroxide, TBHQ, Phenylpropanol, Titanium Dioxide (CI 77891)
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 ML Bottle Carton
-
INGREDIENTS AND APPEARANCE
DAILY ANTIOXIDANT MOISTURIZER SPF 30. SPF 30 BROAD SPECTRUM SUNSCREEN UVA-UVB
avobenzone, octocrylene, and octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-580 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 27.5 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Butyloctyl Salicylate (UNII: 2EH13UN8D3) Glycerin (UNII: PDC6A3C0OX) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) Dimethicone (UNII: 92RU3N3Y1O) CORN OIL (UNII: 8470G57WFM) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) COCOYL CAPRYLOCAPRATE (UNII: 8D9H4QU99H) Polysilicone-15 (UNII: F8DRP5BB29) Sodium Stearoyl Glutamate (UNII: 65A9F4P024) PANTHENOL (UNII: WV9CM0O67Z) VITIS VINIFERA SEED (UNII: C34U15ICXA) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ALOE VERA LEAF (UNII: ZY81Z83H0X) Caffeine (UNII: 3G6A5W338E) Proline (UNII: 9DLQ4CIU6V) Phytosphingosine (UNII: GIN46U9Q2Q) Ceramide AP (UNII: F1X8L2B00J) CERAMIDE 9 (UNII: 88KCS7120E) Ceramide NP (UNII: 4370DF050B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Allantoin (UNII: 344S277G0Z) CHAMOMILE (UNII: FGL3685T2X) Niacinamide (UNII: 25X51I8RD4) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) MACADAMIA OIL (UNII: 515610SU8C) OLIVE OIL (UNII: 6UYK2W1W1E) Rosa Moschata Seed Oil (UNII: T031ZE559T) LAVENDER OIL (UNII: ZBP1YXW0H8) Diatomaceous Earth (UNII: 2RF6EJ0M85) LAMINARIA DIGITATA (UNII: 15E7C67EE8) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) TEA TREE OIL (UNII: VIF565UC2G) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Caprylyl Glycol (UNII: 00YIU5438U) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) SESAME OIL (UNII: QX10HYY4QV) ROSEMARY (UNII: IJ67X351P9) Teprenone (UNII: S8S8451A4O) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) Mica (UNII: V8A1AW0880) Sorbitol (UNII: 506T60A25R) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) Caprylhydroxamic Acid (UNII: UPY805K99W) Ethylhexylglycerin (UNII: 147D247K3P) Cholesterol (UNII: 97C5T2UQ7J) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) Xanthan Gum (UNII: TTV12P4NEE) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) Sodium Hydroxide (UNII: 55X04QC32I) TERT-BUTYLHYDROQUINONE (UNII: C12674942B) PHENYLPROPANOL (UNII: 0F897O3O4M) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-580-01 1 in 1 CARTON 07/01/2023 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/01/2023 Labeler - MDSolarSciences (013647301)