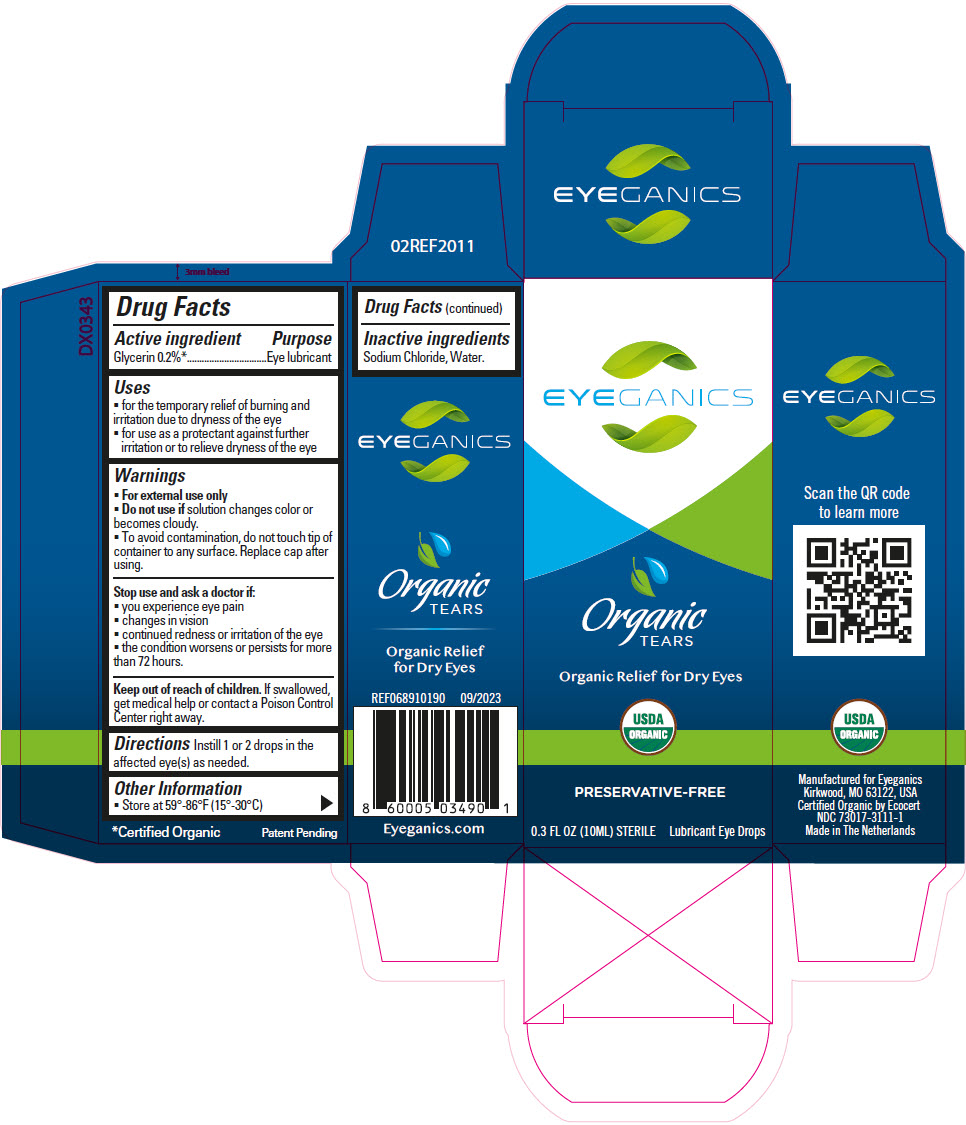
Label: EYEGANICS ORGANIC TEARS- glycerin solution/ drops
- NDC Code(s): 73017-3111-1
- Packager: Eyeganics LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 10 ML Bottle Carton
-
INGREDIENTS AND APPEARANCE
EYEGANICS ORGANIC TEARS
glycerin solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73017-3111 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Glycerin (UNII: PDC6A3C0OX) (Glycerin - UNII:PDC6A3C0OX) Glycerin 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73017-3111-1 1 in 1 CARTON 05/13/2019 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M018 05/13/2019 Labeler - Eyeganics LLC (084011651)