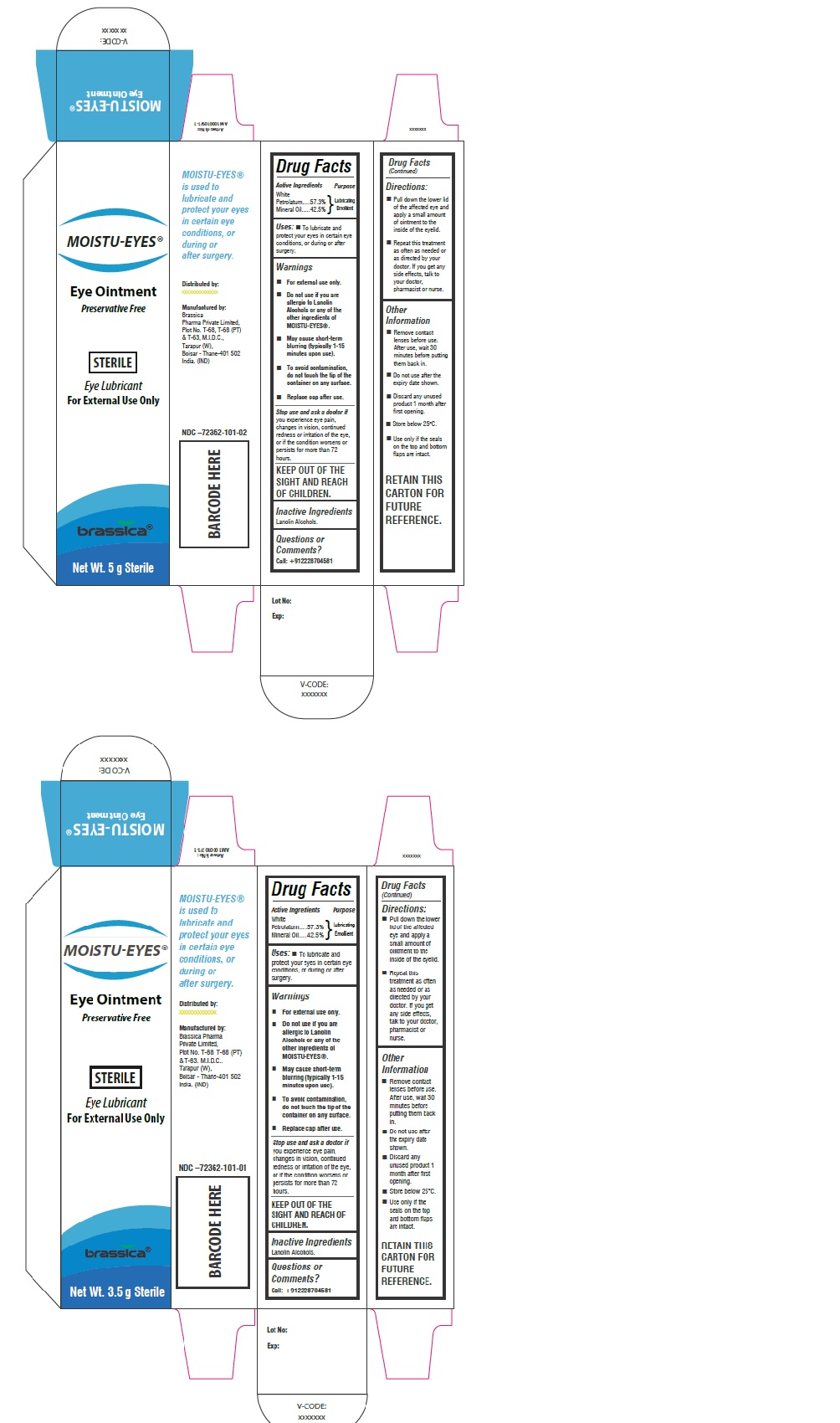
Label: MOISTU-EYES- white petrolatum, mineral oil ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 72362-101-01, 72362-101-02 - Packager: BRASSICA PHARMA PRIVATE LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 28, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Ingredients:
- Purpose
- Uses:
-
Warning
- For external use only.
- Do not use if you are allergic to Lanolin Alcohols or any of the other ingredients of MOISTU-EYES ®.
- May cause short-term blurring (typically 1-15 minutes upon use).
Do not drive or use machinery unless your vision is clear.
Stop use and ask a doctor if
you experience any of the following, stop use and see your doctor: eye pain, eye irritation, eye redness, eye itching, swelling of the eyelid, increased tear production, feeling there's something in your eye, hypersensitivity (allergic reaction).
- KEEP OUT OF REACH OF CHILDREN
- Directions:
- Other Information
- Inactive Ingredients:
- Question or Comments:
-
SPL UNCLASSIFIED SECTION
Eye Ointment
Preservative FreeSTERILE
Eye Lubricant
For External UseMoistueyes ® is used to lubricate and protect your eyes in certain eye conditions, or during or after surgery.
Distributed by:
Amilco Ltd, Mckenzie House, Bury Street, Ruislip, HA4 7TL, UKManufactured by:
Brassica Pharma Private Limited,
Plot No.T-68, T-68 (PT) & T-63, M.I.D.C., Tarapur, (W), Boisar - Thane-401 502 India. (IND) - Packaging
-
INGREDIENTS AND APPEARANCE
MOISTU-EYES
white petrolatum, mineral oil ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72362-101 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 57.3 g in 100 g MINERAL OIL (UNII: T5L8T28FGP) (MINERAL OIL - UNII:T5L8T28FGP) MINERAL OIL 42.5 g in 100 g Inactive Ingredients Ingredient Name Strength LANOLIN ALCOHOLS (UNII: 884C3FA9HE) Product Characteristics Color white (Off white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72362-101-01 1 in 1 CARTON 06/11/2018 1 3.5 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:72362-101-02 1 in 1 CARTON 06/11/2018 2 5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 06/11/2018 Labeler - BRASSICA PHARMA PRIVATE LIMITED (675476593) Establishment Name Address ID/FEI Business Operations BRASSICA PHARMA PRIVATE LIMITED 675476593 manufacture(72362-101)