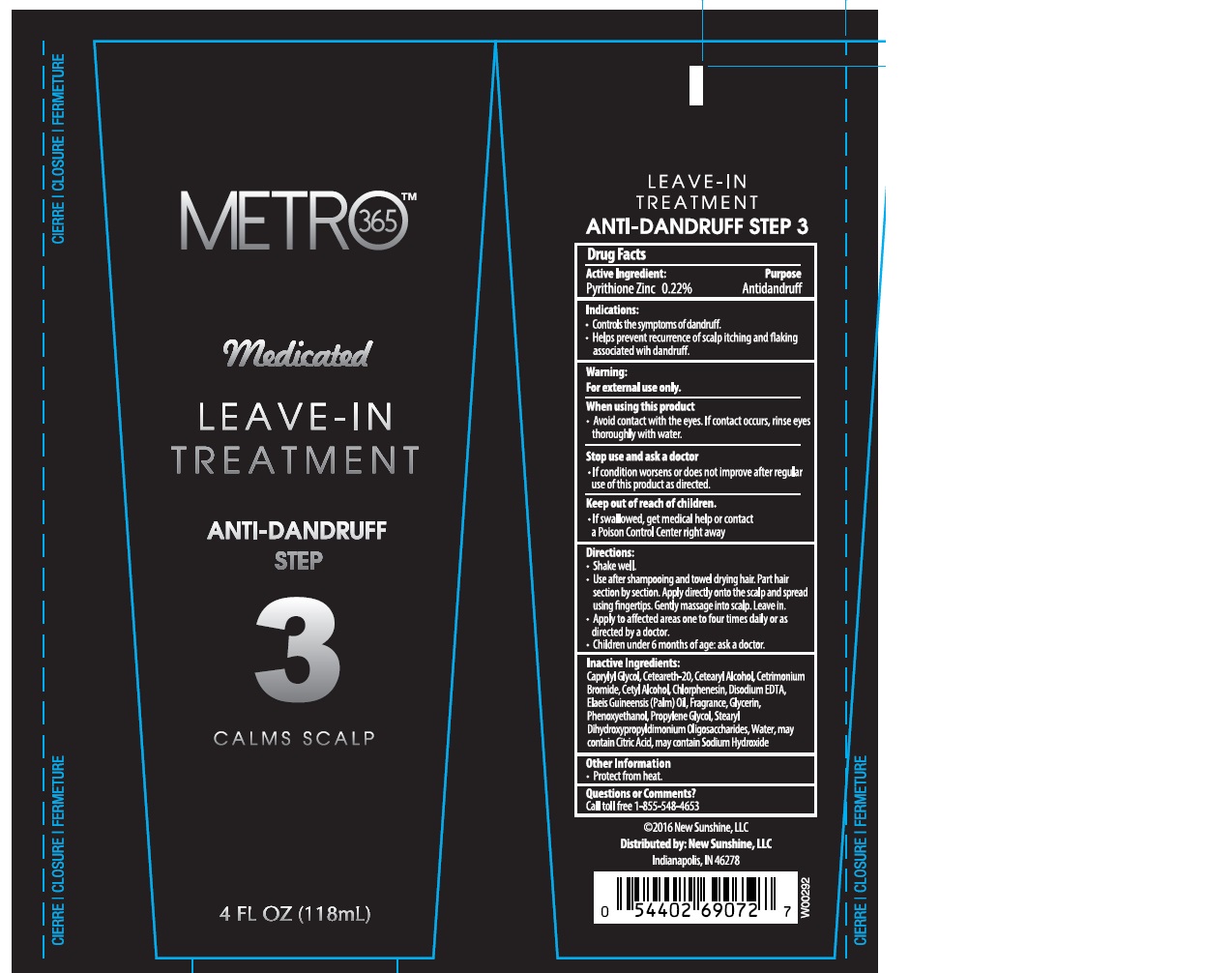
Label: AUSTRALIAN GOLD METRO 365 MEDICATED LEAVE-IN TREATMENT STEP 3- pyrithione zinc 0.22% cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 58443-0243-3 - Packager: Prime Enterprises Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Indications
- Warnings
-
Directions
- Shake well
- Use after shampooing and towel drying hair. Part hair section by section. Apply directly onto the scalp and spread using fingertips. Gently massage into scalp. Leave in.
- Apply to affected areas one to four times daily or as directed by a doctor.
- Children under 6 months of age: ask a doctor.
-
INACTIVE INGREDIENT
Caprylyl Glycol, Ceteareth-20, Cetearyl Alcohol, Cetrimonium Bromide, Cetyl Alcohol, Chlorphenesin, Disodium EDTA, Elaeis Guineensis (Palm) Oil, Fragrance, Glycerin, Phenoxyethanol, Propylene Glycol, Stearyl Dihydroxypropyldimonium Oligosaccharides, Water, may contain Citric Acid, may contain Sodium Hydroxide
- Other Information
- Questions or Comments?
- Australian Gold Metro 365 Medicated Leave-In Treatment Step 3
-
INGREDIENTS AND APPEARANCE
AUSTRALIAN GOLD METRO 365 MEDICATED LEAVE-IN TREATMENT STEP 3
pyrithione zinc 0.22% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58443-0243 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 2.14 mg in 1 mL Inactive Ingredients Ingredient Name Strength CHLORPHENESIN (UNII: I670DAL4SZ) CETRIMONIUM BROMIDE (UNII: L64N7M9BWR) PALM OIL (UNII: 5QUO05548Z) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM HYDROXIDE (UNII: 55X04QC32I) CETYL ALCOHOL (UNII: 936JST6JCN) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) WATER (UNII: 059QF0KO0R) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58443-0243-3 118 mL in 1 TUBE; Type 0: Not a Combination Product 10/12/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 10/12/2016 Labeler - Prime Enterprises Inc. (101946028) Registrant - Prime Enterprises Inc. (101946028) Establishment Name Address ID/FEI Business Operations Prime Enterprises Inc. 101946028 label(58443-0243) , manufacture(58443-0243) , analysis(58443-0243) , pack(58443-0243)