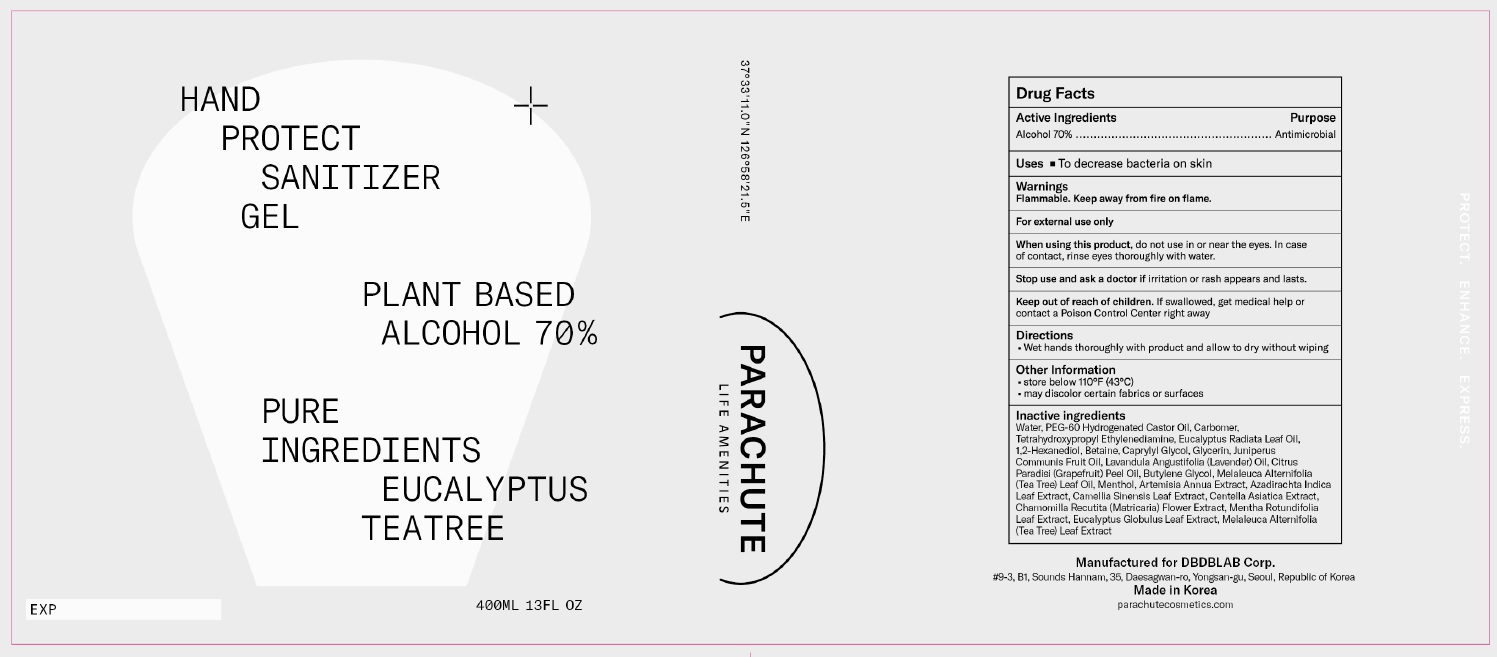
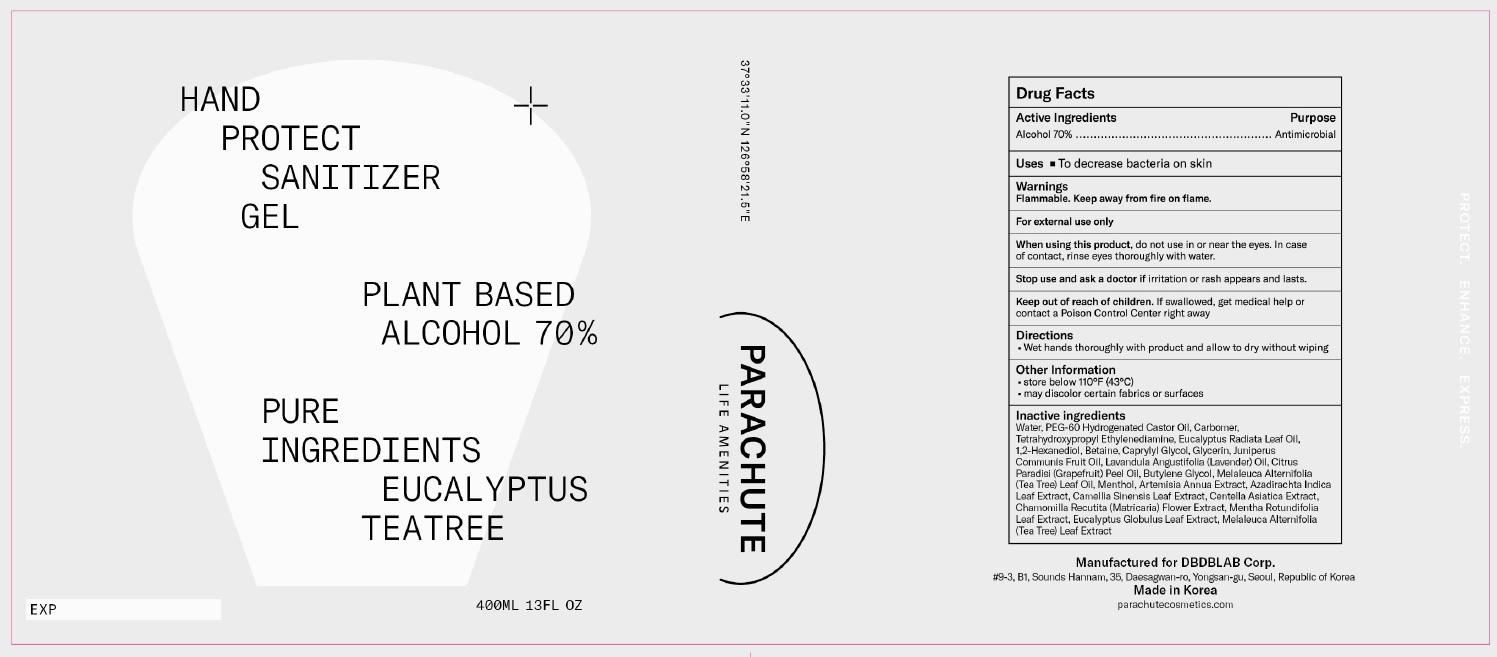
Label: PARACHUTE HAND PROTECT GEL- alcohol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 81135-001-01, 81135-001-02 - Packager: DBDBLAB corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 18, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
-
Inactive Ingredients:
Water, PEG-60 Hydrogenated Castor Oil, Carbomer, Tetrahydroxypropyl Ethylenediamine, Eucalyptus Radiata Leaf Oil, 1,2-Hexanediol, Betaine, Caprylyl Glycol, Glycerin, Juniperus Communis Fruit Oil, Lavandula Angustifolia (Lavender) Oil, Citrus Paradisi (Grapefruit) Peel Oil, Butylene Glycol, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Menthol, Artemisia Annua Extract, Azadirachta Indica Leaf Extract, Camellia Sinensis Leaf Extract, Centella Asiatica Extract, Chamomilla Recutita (Matricaria) Flower Extract, Mentha Rotundifolia Leaf Extract, Eucalyptus Globulus Leaf Extract, Melaleuca Alternifolia (Tea Tree) Leaf Extract
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PARACHUTE HAND PROTECT GEL
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81135-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) EDETOL (UNII: Q4R969U9FR) EUCALYPTUS OIL (UNII: 2R04ONI662) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) BETAINE (UNII: 3SCV180C9W) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCERIN (UNII: PDC6A3C0OX) JUNIPER BERRY OIL (UNII: SZH16H44UY) GRAPEFRUIT OIL (UNII: YR377U58W9) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAVENDER OIL (UNII: ZBP1YXW0H8) TEA TREE OIL (UNII: VIF565UC2G) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) AZADIRACHTA INDICA LEAF (UNII: HKY915780T) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CENTELLA ASIATICA WHOLE (UNII: 7M867G6T1U) CHAMOMILE (UNII: FGL3685T2X) MENTHA SUAVEOLENS LEAF (UNII: 1341ZC68MK) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81135-001-02 1 in 1 CARTON 01/18/2021 1 NDC:81135-001-01 400 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/18/2021 Labeler - DBDBLAB corp. (695584806) Establishment Name Address ID/FEI Business Operations Ecoment 688485136 manufacture(81135-001)