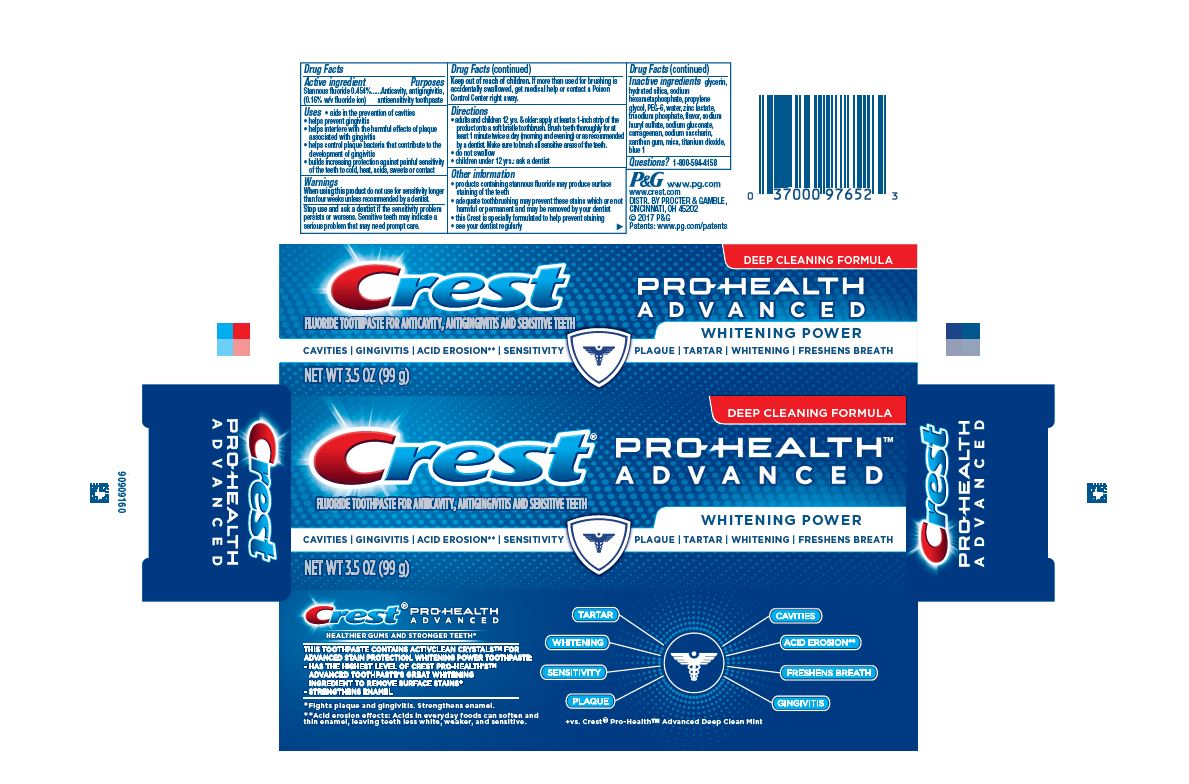
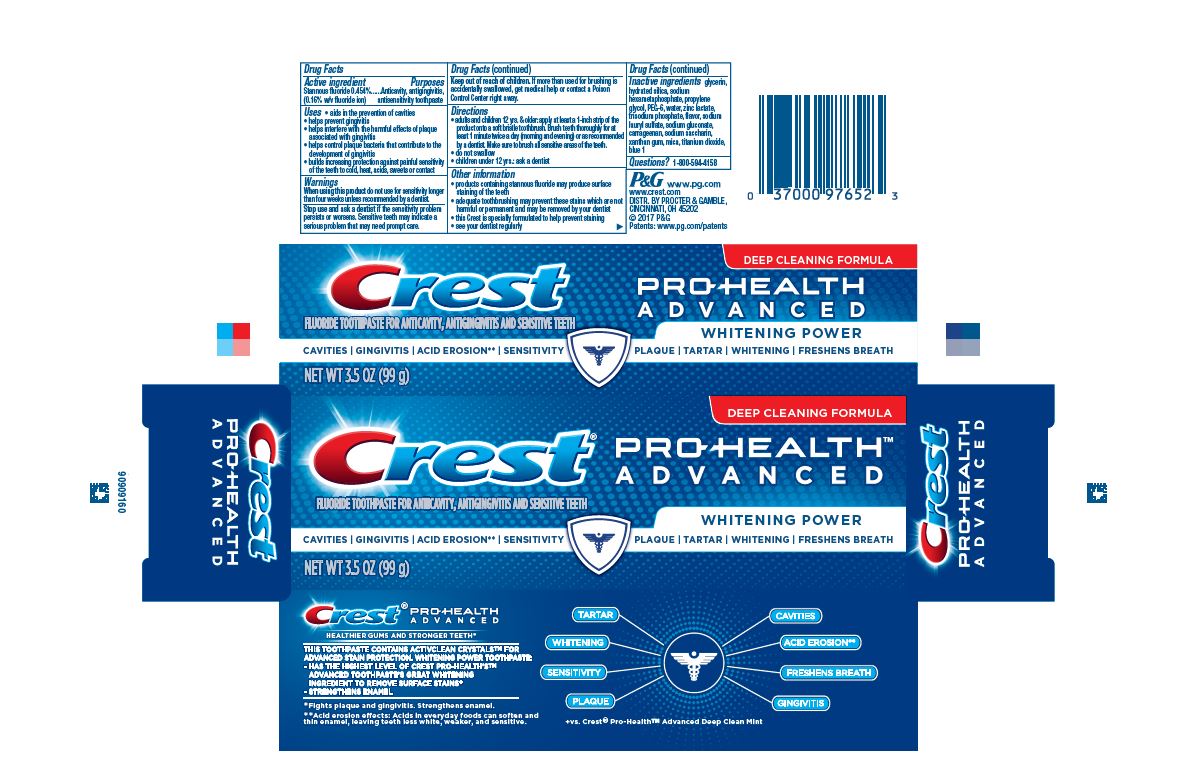
Label: CREST PRO-HEALTH EXTRA WHITENING POWER- stannous fluoride gel, dentifrice
-
NDC Code(s):
37000-885-03,
37000-885-05,
37000-885-07,
37000-885-35, view more37000-885-51
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purposes
-
Uses
- aids in the prevention of cavities
- helps prevent gingivitis
- helps interfere with the harmful effects of plaque associated with gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets, or contact
- Warnings
-
Directions
- adults and children 12 yrs. & older: apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist. Make sure to brush all sensitive areas of the teeth.
- do not swallow
- children under 12 yrs.: ask a dentist
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 144 g Tube Carton
-
INGREDIENTS AND APPEARANCE
CREST PRO-HEALTH EXTRA WHITENING POWER
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37000-885 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.6 mg in 1 g Inactive Ingredients Ingredient Name Strength SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) WATER (UNII: 059QF0KO0R) ZINC LACTATE (UNII: 2GXR25858Y) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM GLUCONATE (UNII: R6Q3791S76) CARRAGEENAN (UNII: 5C69YCD2YJ) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM PHOSPHATE, TRIBASIC, ANHYDROUS (UNII: SX01TZO3QZ) MICA (UNII: V8A1AW0880) GLYCERIN (UNII: PDC6A3C0OX) HYDRATED SILICA (UNII: Y6O7T4G8P9) XANTHAN GUM (UNII: TTV12P4NEE) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue Score Shape Size Flavor WINTERGREEN Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37000-885-03 1 in 1 CARTON 01/01/2016 02/07/2019 1 104 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:37000-885-05 1 in 1 CARTON 01/01/2016 02/07/2019 2 144 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:37000-885-07 1 in 1 CARTON 01/01/2016 02/07/2019 3 198 g in 1 TUBE; Type 0: Not a Combination Product 4 NDC:37000-885-35 1 in 1 CARTON 01/01/2016 4 99 g in 1 TUBE; Type 0: Not a Combination Product 5 NDC:37000-885-51 1 in 1 CARTON 01/01/2016 5 144 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 01/01/2016 Labeler - The Procter & Gamble Manufacturing Company (004238200)