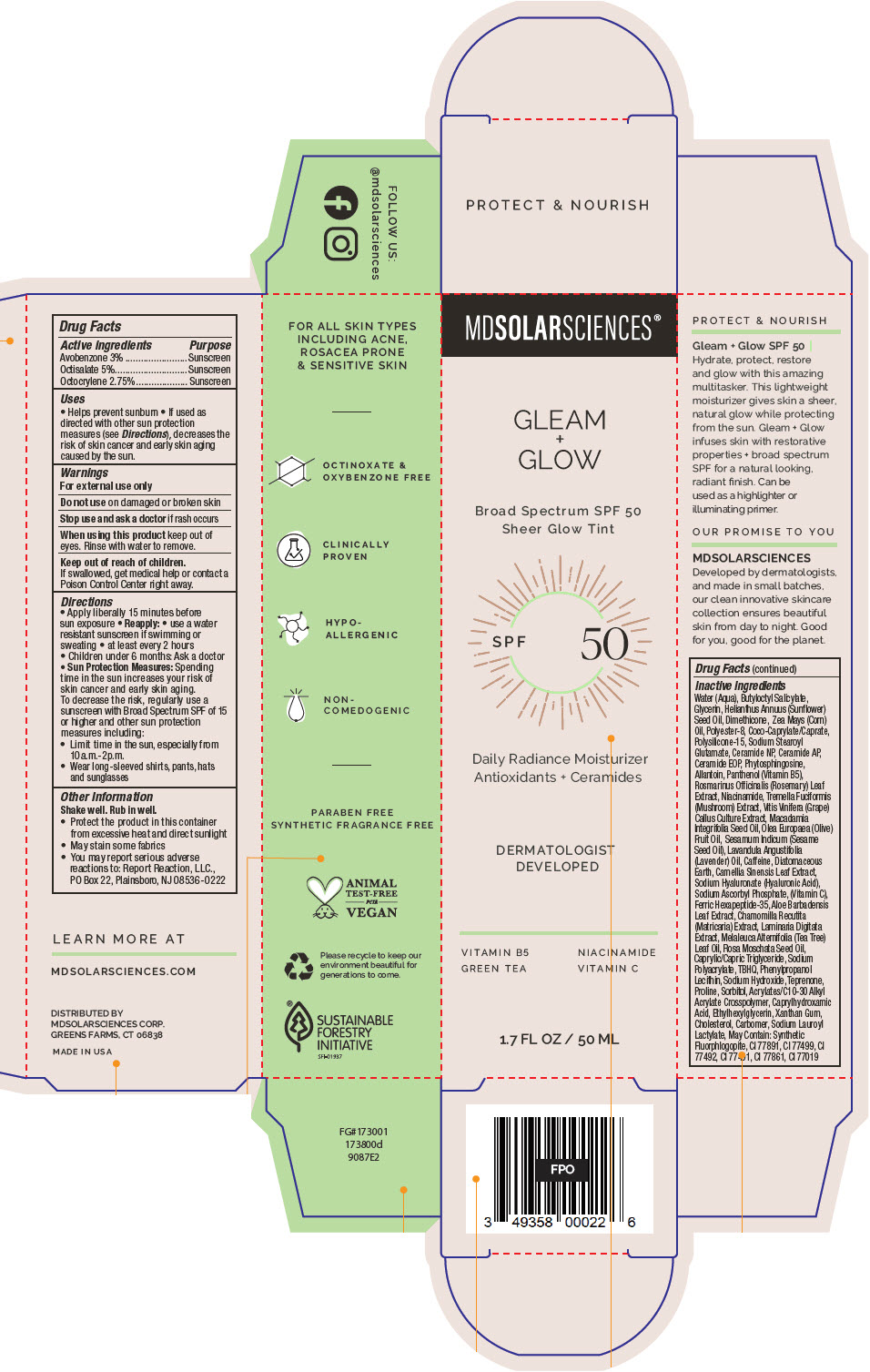
Label: GLEAM PLUS GLOW SPF 50. SPF 50 BROAD SPECTRUM SUNSCREEN UVA-UVB SHEER GLOW TINT- avobenzone, octisalate, and octocrylene lotion
- NDC Code(s): 49358-581-01
- Packager: MDSolarSciences
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
-
Reapply:
- use a water resistant sunscreen if swimming or sweating
- at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10a.m.-2p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other Information
-
Inactive Ingredients
Water (Aqua), Butyloctyl Salicylate, Glycerin, Helianthus Annuus (Sunflower) Seed Oil, Dimethicone, Zea Mays (Corn) Oil, Polyester-8, Coco-Caprylate/Caprate, Polysilicone-15, Sodium Stearoyl Glutamate, Ceramide NP, Ceramide AP, Ceramide EOP, Phytosphingosine, Allantoin, Panthenol (Vitamin B5), Rosmarinus Officinalis (Rosemary) Leaf Extract, Niacinamide, Tremella Fuciformis (Mushroom) Extract, Vitis Vinifera (Grape) Callus Culture Extract, Macadamia Integrifolia Seed Oil, Olea Europaea (Olive) Fruit Oil, Sesamum Indicum (Sesame Seed Oil), Lavandula Angustifolia (Lavender) Oil, Caffeine, Diatomaceous Earth, Camellia Sinensis Leaf Extract, Sodium Hyaluronate (Hyaluronic Acid), Sodium Ascorbyl Phosphate, (Vitamin C), Ferric Hexapeptide-35, Aloe Barbadensis Leaf Extract, Chamomilla Recutita (Matricaria) Extract, Laminaria Digitata Extract, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Rosa Moschata Seed Oil, Caprylic/Capric Triglyceride, Sodium Polyacrylate, TBHQ, Phenylpropanol Lecithin, Sodium Hydroxide, Teprenone, Proline, Sorbitol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylhydroxamic Acid, Ethylhexylglycerin, Xanthan Gum, Cholesterol, Carbomer, Sodium Lauroyl Lactylate, May Contain: Synthetic Fluorphlogopite, CI 77891, CI 77499, CI 77492, CI 77491, CI 77861, CI 77019
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 ML Bottle Carton
-
INGREDIENTS AND APPEARANCE
GLEAM PLUS GLOW SPF 50. SPF 50 BROAD SPECTRUM SUNSCREEN UVA-UVB SHEER GLOW TINT
avobenzone, octisalate, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-581 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 27.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Butyloctyl Salicylate (UNII: 2EH13UN8D3) Glycerin (UNII: PDC6A3C0OX) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) Dimethicone (UNII: 92RU3N3Y1O) CORN OIL (UNII: 8470G57WFM) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) COCOYL CAPRYLOCAPRATE (UNII: 8D9H4QU99H) Polysilicone-15 (UNII: F8DRP5BB29) Sodium Stearoyl Glutamate (UNII: 65A9F4P024) Ceramide NP (UNII: 4370DF050B) Ceramide AP (UNII: F1X8L2B00J) CERAMIDE 9 (UNII: 88KCS7120E) Phytosphingosine (UNII: GIN46U9Q2Q) Allantoin (UNII: 344S277G0Z) PANTHENOL (UNII: WV9CM0O67Z) ROSEMARY (UNII: IJ67X351P9) Niacinamide (UNII: 25X51I8RD4) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) VITIS VINIFERA SEED (UNII: C34U15ICXA) MACADAMIA OIL (UNII: 515610SU8C) OLIVE OIL (UNII: 6UYK2W1W1E) SESAME OIL (UNII: QX10HYY4QV) LAVENDER OIL (UNII: ZBP1YXW0H8) Caffeine (UNII: 3G6A5W338E) Diatomaceous Earth (UNII: 2RF6EJ0M85) GREEN TEA LEAF (UNII: W2ZU1RY8B0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHAMOMILE (UNII: FGL3685T2X) LAMINARIA DIGITATA (UNII: 15E7C67EE8) TEA TREE OIL (UNII: VIF565UC2G) Rosa Moschata Seed Oil (UNII: T031ZE559T) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) TERT-BUTYLHYDROQUINONE (UNII: C12674942B) PHENYLPROPANOL (UNII: 0F897O3O4M) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) Sodium Hydroxide (UNII: 55X04QC32I) Teprenone (UNII: S8S8451A4O) Proline (UNII: 9DLQ4CIU6V) Sorbitol (UNII: 506T60A25R) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) Caprylhydroxamic Acid (UNII: UPY805K99W) Ethylhexylglycerin (UNII: 147D247K3P) Xanthan Gum (UNII: TTV12P4NEE) Cholesterol (UNII: 97C5T2UQ7J) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) May contain TITANIUM DIOXIDE (UNII: 15FIX9V2JP) May contain FERRIC OXIDE YELLOW (UNII: EX438O2MRT) May contain FERRIC OXIDE RED (UNII: 1K09F3G675) May contain STANNIC OXIDE (UNII: KM7N50LOS6) May contain FERROSOFERRIC OXIDE (UNII: XM0M87F357) May contain MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-581-01 1 in 1 CARTON 07/01/2023 1 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/01/2023 Labeler - MDSolarSciences (013647301)