Label: SKIN FIRM LIPOAMINO HYDRATOR SPF 15 MOLTON BROWN- avobenzone, ensulizole, octinoxate, octocrylene lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 68814-250-01, 68814-250-02 - Packager: Molton Brown LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 4, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
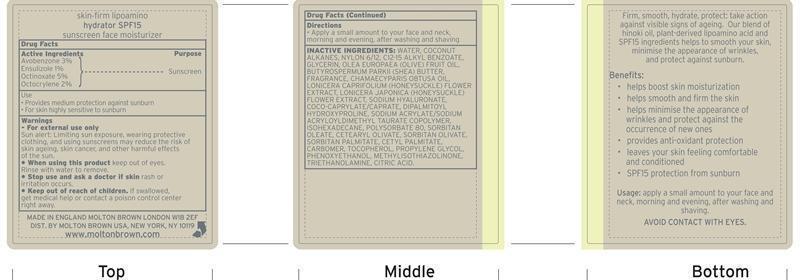
Active Ingredients Purpose
Avobenzone 3% Sunscreen
Ensulizole 1% Sunscreen
Octinoxate 5% Sunscreen
Octocrylene 2% Sunscreen
warnings
For external use only
Sun alert: Limiting sun exposure, wearing protective clothing and using sunscreens may reduce the risk of skin ageing, skin cancer, and other harmful effects of the sun.
When using this product: Keep out of eyes. Rinse with water to remove.
If swallowed, get medical help or contact a poison control center right away.
Directions
Apply a small amount to your face and neck, morning and evening, after washing and shaving.
Inactive Ingredients
water, coconut alkanes, nylon 6/12, C12-15 alkyl benzoate, glycerin, olea europaea (olive) fruit oil, butyrospermum parkii (shea) butter, fragrance, chamaecyparis obtusa oil, lonicera caprifolium (honeysuckle) flower extract, lonicera japonica (honeysuckle) flower extract, sodium hyaluronate, coco-caprylate/caprate, dipalmitoyl hydroxyproline, sodium acrylate/sodium acryloyldimethyl taurate copolymer, isohexadecane, polysorbate 80, sorbitan oleate, cetearyl olivate, sorbitan palmitate, cetyl palmitate, carbomer, tocopherol, propylene glycol, phenoxyethanol, methylisothiazolinone, thriethanolamine, citric acid.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKIN FIRM LIPOAMINO HYDRATOR SPF 15 MOLTON BROWN
avobenzone, ensulizole, octinoxate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68814-250 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 1 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCONUT ALKANES (UNII: 1E5KJY107T) ENSULIZOLE (UNII: 9YQ9DI1W42) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERIN (UNII: PDC6A3C0OX) OLIVE OIL (UNII: 6UYK2W1W1E) SHEA BUTTER (UNII: K49155WL9Y) CHAMAECYPARIS OBTUSA WOOD OIL (UNII: P2OMP71Y62) LONICERA CAPRIFOLIUM FLOWER (UNII: 5N1WD9784U) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) HYALURONATE SODIUM (UNII: YSE9PPT4TH) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) ISOHEXADECANE (UNII: 918X1OUF1E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) CETEARYL OLIVATE (UNII: 58B69Q84JO) SORBITAN OLIVATE (UNII: MDL271E3GR) SORBITAN MONOPALMITATE (UNII: 77K6Z421KU) CETYL PALMITATE (UNII: 5ZA2S6B08X) CARBOMER 934 (UNII: Z135WT9208) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) TROLAMINE (UNII: 9O3K93S3TK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68814-250-02 1 in 1 CARTON 1 NDC:68814-250-01 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/01/2011 Labeler - Molton Brown LTD (769334822) Registrant - Molton Brown LTD (769334822) Establishment Name Address ID/FEI Business Operations DDD LTD 216339804 manufacture(68814-250)