Label: BACTINE MAX- bactine max wound wash liquid
- NDC Code(s): 65197-820-80
- Packager: WellSpring Pharmaceutical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 29, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
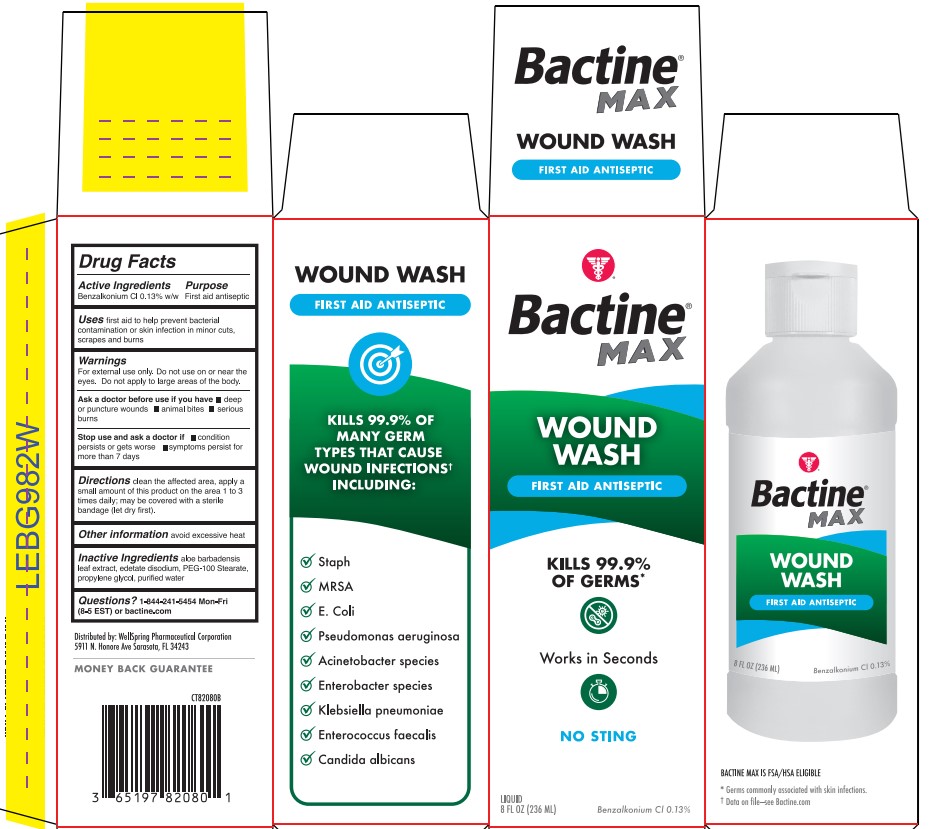
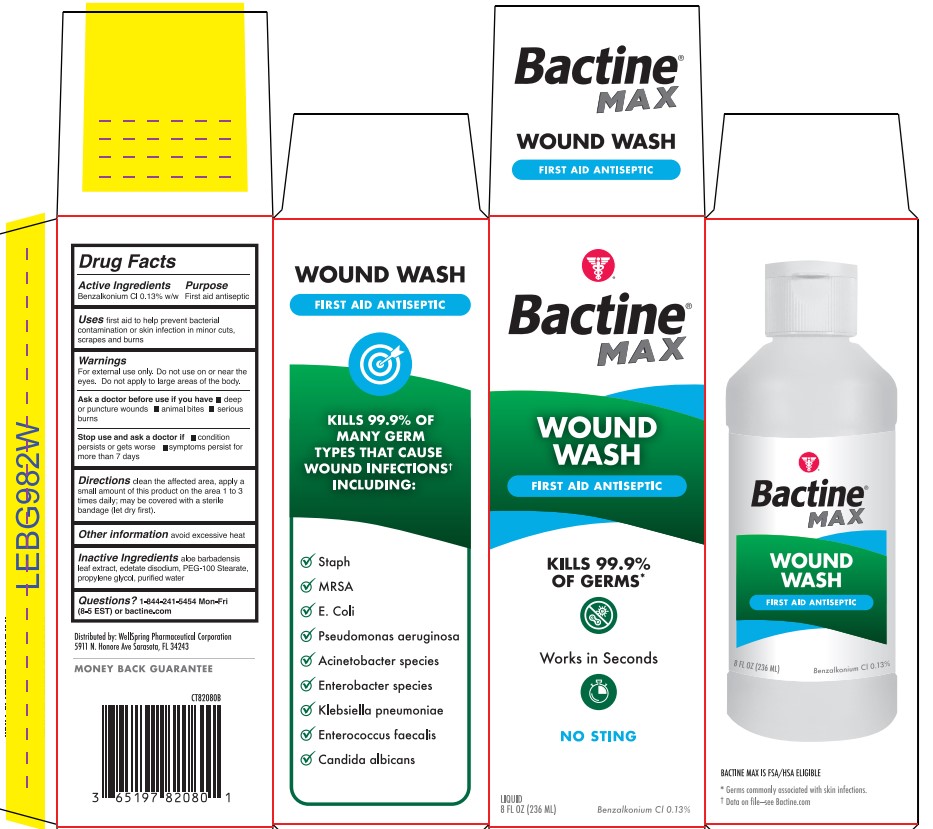
- Active ingredient
- Purpose
- Use
- Warning
- Directions
- Other information
- Inactive ingredients
- Questions?
- Bactine Wound Wash Label
-
INGREDIENTS AND APPEARANCE
BACTINE MAX
bactine max wound wash liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65197-820 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) PEG-100 STEARATE (UNII: YD01N1999R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65197-820-80 1 in 1 CARTON 04/15/2023 1 236 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 04/15/2023 Labeler - WellSpring Pharmaceutical Corporation (110999054)