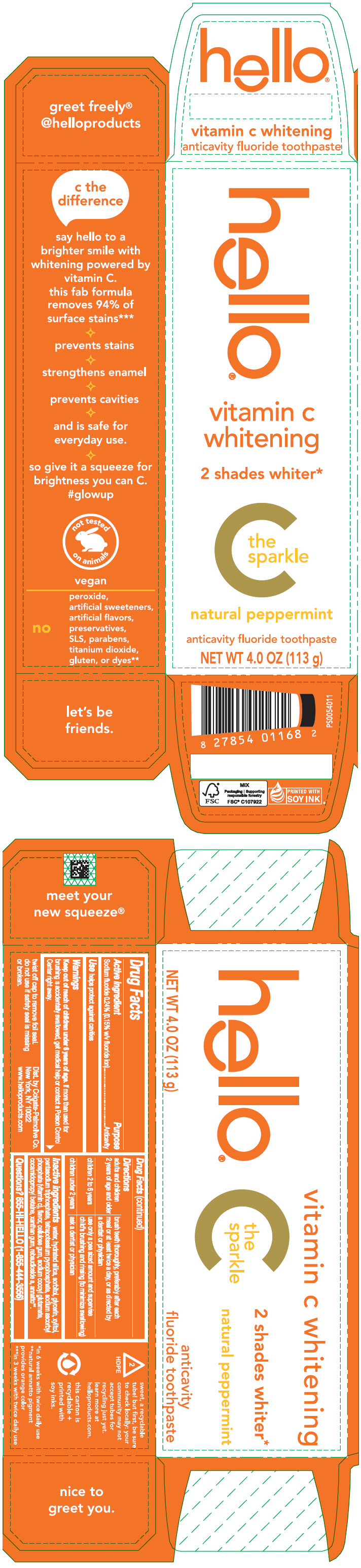
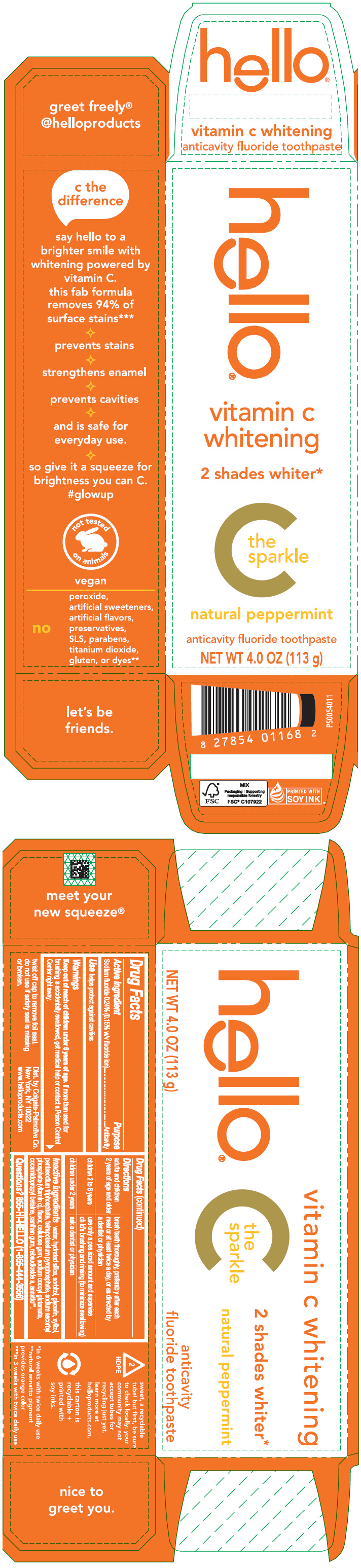
Label: HELLO VITAMIN C WHITENING- sodium fluoride paste, dentifrice
- NDC Code(s): 35000-689-10
- Packager: Colgate-Palmolive Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
adults and children 2 years of age and older brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician chldren 2 to 6 years use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) children under 2 years ask a dentist or physician -
Inactive ingredients
water, hydrated silica, sorbitol, glycerin, xylitol, pentasodium triphosphate, tetrapotassium pyrophosphate, sodium ascorbyl phosphate (vitamin c), flavor, cellulose gum, sodium cocoyl glutamate, cocamidopropyl betaine, xanthan gum, rebaudioside a, annatto1.
- 1
- natural annatto pigment provides orange color
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 113 g Tube Carton
-
INGREDIENTS AND APPEARANCE
HELLO VITAMIN C WHITENING
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35000-689 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) XYLITOL (UNII: VCQ006KQ1E) SODIUM TRIPOLYPHOSPHATE ANHYDROUS (UNII: 9SW4PFD2FZ) SODIUM PYROPHOSPHATE (UNII: O352864B8Z) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SODIUM COCOYL GLUTAMATE (UNII: BMT4RCZ3HG) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) XANTHAN GUM (UNII: TTV12P4NEE) REBAUDIOSIDE A (UNII: B3FUD0528F) ANNATTO (UNII: 6PQP1V1B6O) Product Characteristics Color ORANGE Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35000-689-10 1 in 1 CARTON 01/01/2024 1 113 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M021 01/01/2024 Labeler - Colgate-Palmolive Company (001344381)