Label: PROSORIA PSORIASIS TREATMENT- salicylic acid kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 71573-102-01, 71573-117-03 - Packager: Nuvothera, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 8, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
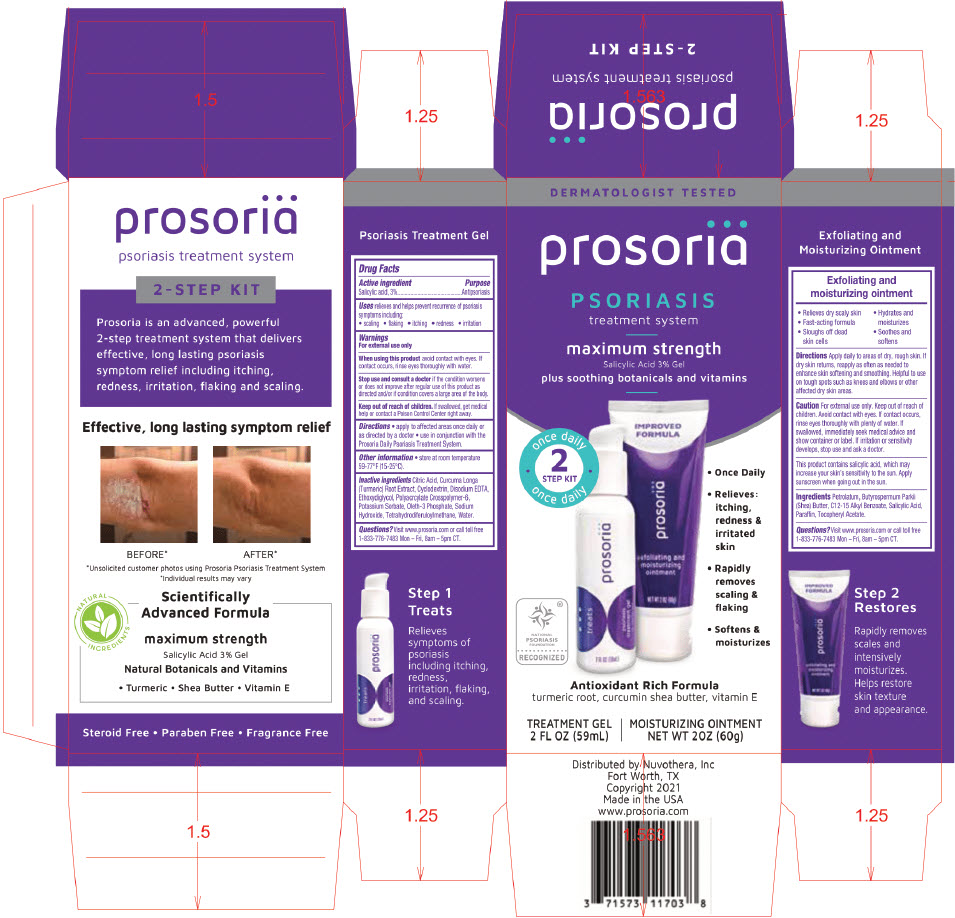
PRINCIPAL DISPLAY PANEL - Kit Carton
DERMATOLOGIST TESTED
prosoriä
PSORIASIS
treatment systemmaximum strength
Salicylic Acid 3% Gelplus soothing botanicals and vitamins
once daily
2
STEP KIT
once dailyNATIONAL
PSORIASIS
FOUNDATION
RECOGNIZED- Once Daily
- Relieves:
itching,
redness &
irritated
skin - Rapidly
removes
scaling &
flaking - Softens &
moisturizes
Antioxidant Rich Formula
turmeric root, curcumin shea butter, vitamin ETREATMENT GEL
2 FL OZ (59mL)MOISTURIZING OINTMENT
NET WT 2OZ (60g)
-
INGREDIENTS AND APPEARANCE
PROSORIA PSORIASIS TREATMENT
salicylic acid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71573-117 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71573-117-03 1 in 1 CARTON 02/18/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 59 mL Part 2 1 TUBE 60 g Part 1 of 2 PROSORIA PSORIASIS TREATMENT
salicylic acid gelProduct Information Item Code (Source) NDC:71573-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 1.77 g in 59 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Diethylene Glycol Monoethyl Ether (UNII: A1A1I8X02B) Tetrahydrodiferuloylmethane (UNII: 00U0645U03) Turmeric (UNII: 856YO1Z64F) Gamma Cyclodextrin (UNII: KZJ0BYZ5VA) Oleth-3 Phosphate (UNII: 8Q0Z18J1VL) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) Sodium Hydroxide (UNII: 55X04QC32I) Potassium Hydroxide (UNII: WZH3C48M4T) Potassium Sorbate (UNII: 1VPU26JZZ4) Citric Acid Monohydrate (UNII: 2968PHW8QP) Product Characteristics Color WHITE (Off White Beige) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71573-102-01 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part358H 11/22/2017 Part 2 of 2 EXFOLIATING AND MOISTURIZING
other skin care preparations ointmentProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Petrolatum (UNII: 4T6H12BN9U) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) INGR Salicylic Acid (UNII: O414PZ4LPZ) INGR Paraffin (UNII: I9O0E3H2ZE) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part358H 02/18/2022 Labeler - Nuvothera, Inc. (080499864) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO 080170933 MANUFACTURE(71573-117) Establishment Name Address ID/FEI Business Operations Global Packaging Systems 964987890 LABEL(71573-117) , PACK(71573-117)