Label: YOUNIQUE BARE YOU BB TINTED MOISTURIZER SUNSCREEN BROAD SPECTRUM SPF-30 DARK DEEP- zinc oxide cream
- NDC Code(s): 71182-0008-1
- Packager: Younique, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
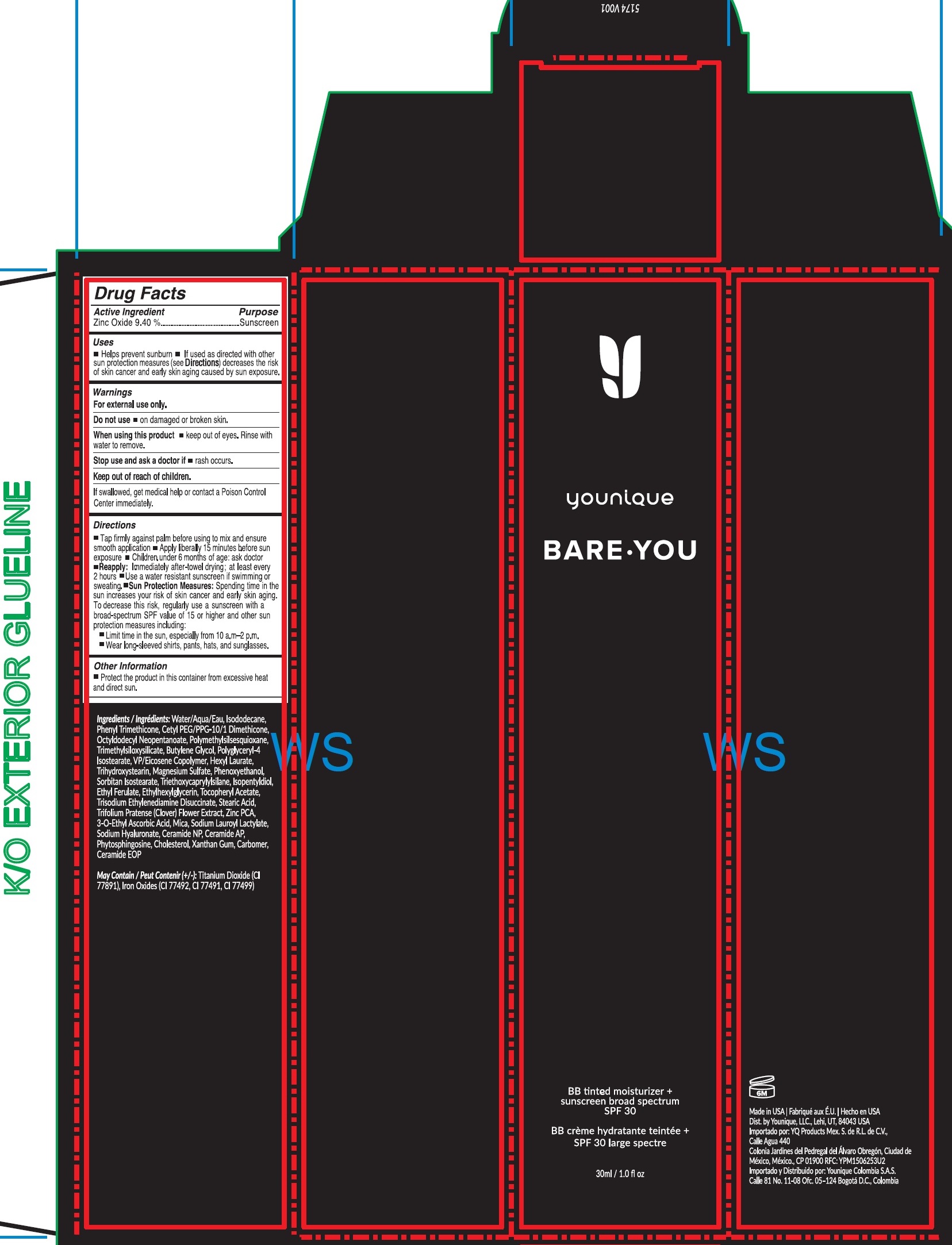
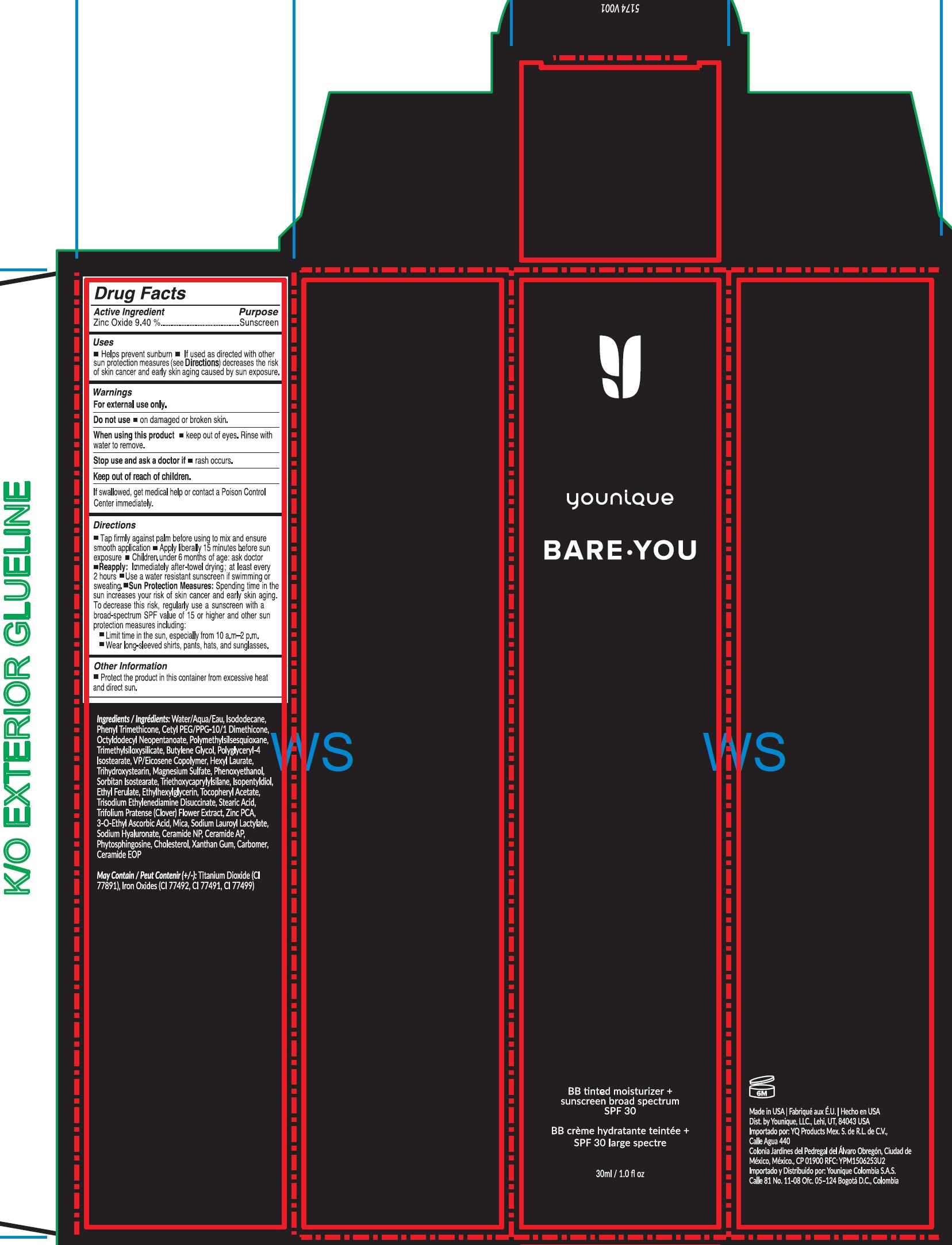
- Drug Facts
- Active Ingredient
- Uses
- Warnings
-
Directions
- Tap firmly against palm before using to mix and ensure smooth application
- Apply liberally 15 minutes before sun exposure
- Children under 6 months of age: ask doctor
- : Immediately after-towel drying; at least every 2 hours Reapply
- Use a water resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regulalry use a sunscreen with a broad-spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures:
- Limit time in the sun, especially from 10 a.m.- 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Other Information
-
Ingredients
Water/Aqua, Isododecane, Phenyl Trimethicone, Cetyl PEG/PPG-10/1 Dimethicone, Octyldodecyl Neopentanoate, Polymethylsilsesquioxane, Trimethylsiloxysilicate, Butylene Glycol, Polyglyceryl-4 Isostearate, VP/Eicosene Copolymer, Hexyl Laurate, Trihydroxystearin, Magnesium Sulfate, Phenoxyethanol, Sorbitan Isostearate, Triethoxycaprylylsilane, Isopentyldiol, Ethyl Ferulate, Ethylhexylglycerin, Tocopheryl Acetate, Trisodium Ethylenediamine Disuccinate, Stearic Acid, Trifolium Pratense (Clover) Flower Extract, Zinc PCA, 3-O-Ethyl Ascorbic Acid, Mica, Sodium Lauroyl Lactylate, Sodium Hyaluronate, Ceramide NP, Ceramide AP, Phytosphingosine, Cholesterol, Xanthan Gum, Carbomer, Ceramide EOP
May Contain (+/-): Titanium Dioxide (CI 77891), Iron Oxides (CI 77492, CI 77491, CI 77499)
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
YOUNIQUE BARE YOU BB TINTED MOISTURIZER SUNSCREEN BROAD SPECTRUM SPF-30 DARK DEEP
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71182-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 94 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ISOPENTYLDIOL (UNII: 19NOL5474Q) ETHYL FERULATE (UNII: 5B8915UELW) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) STEARIC ACID (UNII: 4ELV7Z65AP) TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) ZINC PIDOLATE (UNII: C32PQ86DH4) 3-O-ETHYL ASCORBIC ACID (UNII: 6MW60CB71P) MICA (UNII: V8A1AW0880) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CERAMIDE NP (UNII: 4370DF050B) CERAMIDE AP (UNII: F1X8L2B00J) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CHOLESTEROL (UNII: 97C5T2UQ7J) XANTHAN GUM (UNII: TTV12P4NEE) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71182-0008-1 1 in 1 CARTON 06/01/2023 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2023 Labeler - Younique, LLC (079767410)