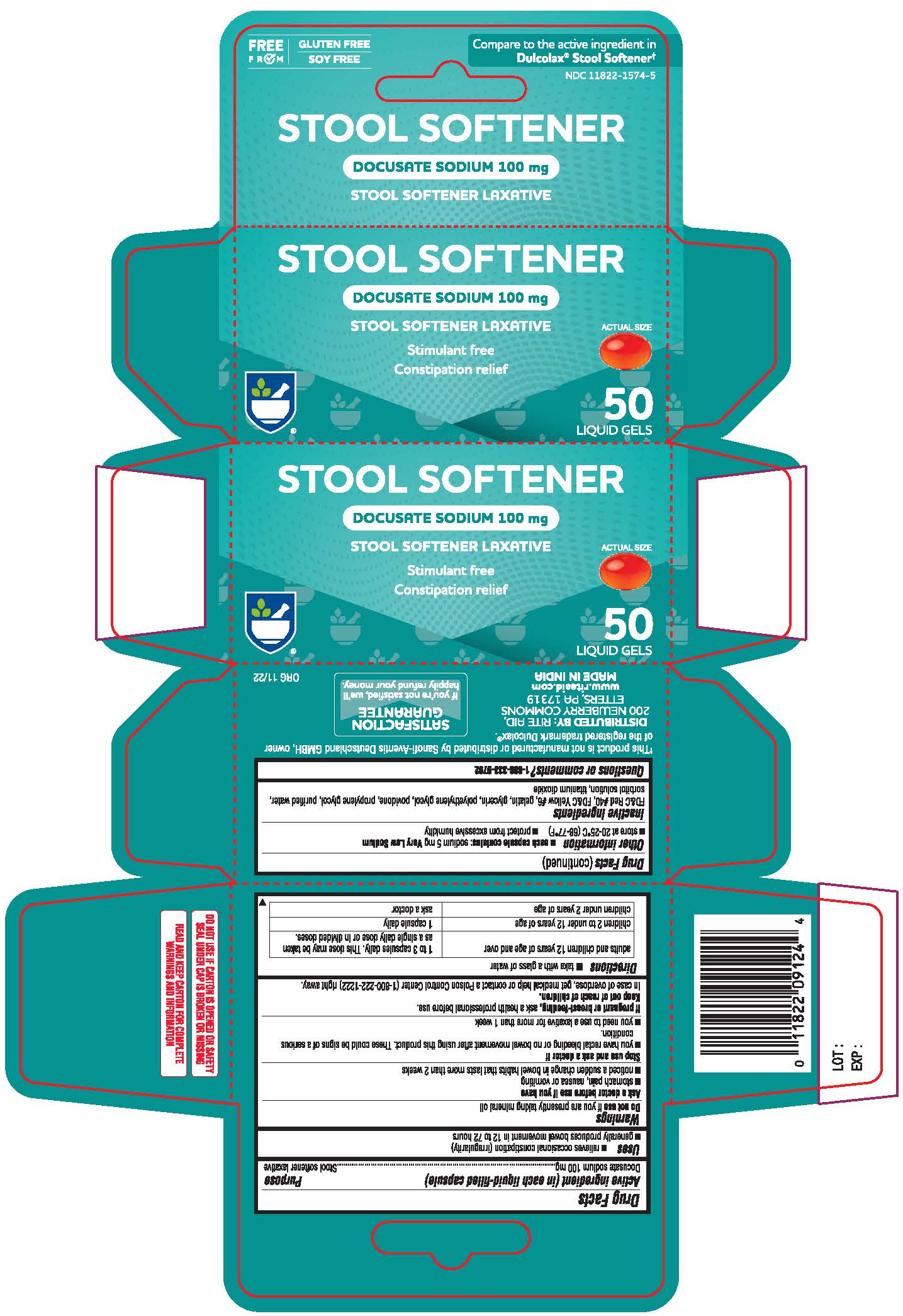
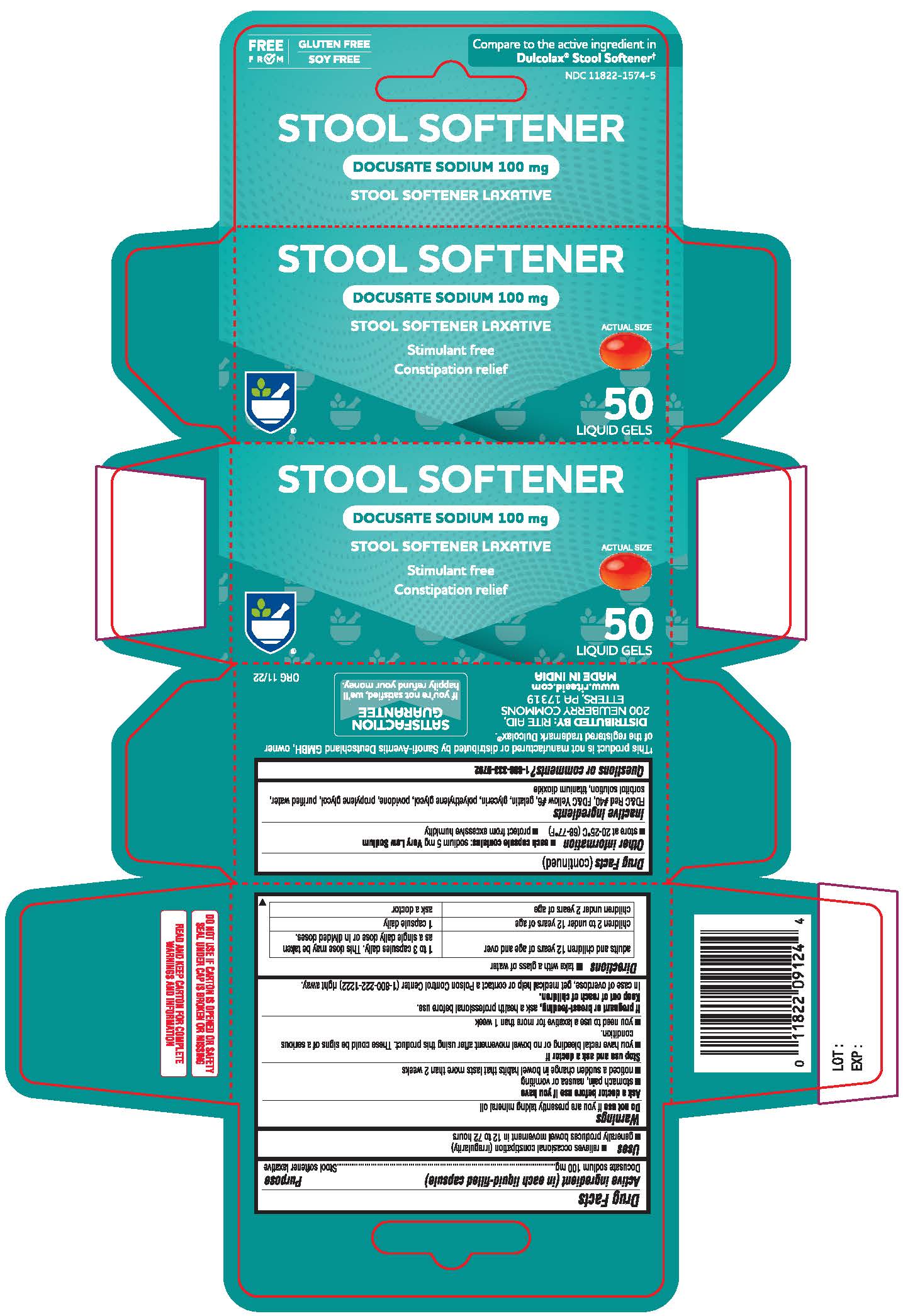
Label: GENTLE STOOL SOFTENER- docusate sodium capsule, liquid filled
- NDC Code(s): 11822-1574-5
- Packager: RITE AID CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are if you have
• stomach pain • nausea • vomiting • noticed a sudden change in bowel habits that lasts over 2 weeks - Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display
-
INGREDIENTS AND APPEARANCE
GENTLE STOOL SOFTENER
docusate sodium capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-1574 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 100 mg Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL SOLUTION (UNII: 8KW3E207O2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape OVAL Size 10mm Flavor Imprint Code 100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-1574-5 1 in 1 CARTON 11/07/2022 1 50 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 11/07/2022 Labeler - RITE AID CORPORATION (014578892)