Label: DEMSER- metyrosine capsule
- NDC Code(s): 25010-305-15
- Packager: Bausch Health US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 1, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
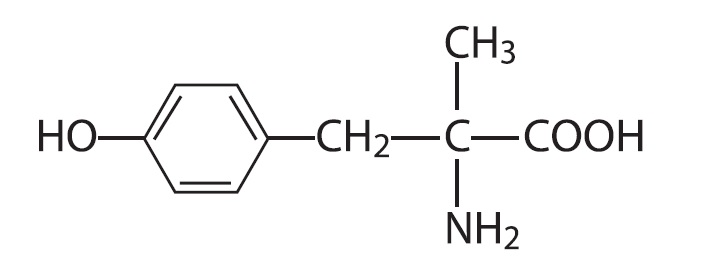
DEMSER (metyrosine) is (–)-α-methyl-L-tyrosine or (α-MPT). It has the following structural formula:
Metyrosine is a white to off-white, crystalline compound of molecular weight 195.22. It is very slightly soluble in water, acetone, and methanol, and insoluble in chloroform and benzene. It is soluble in acidic aqueous solutions. It is also soluble in alkaline aqueous solutions, but is subject to oxidative degradation under these conditions.
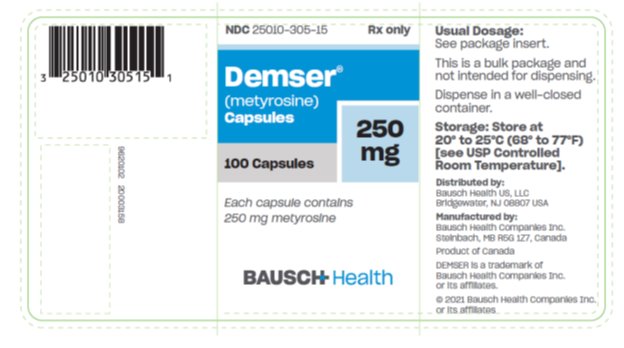
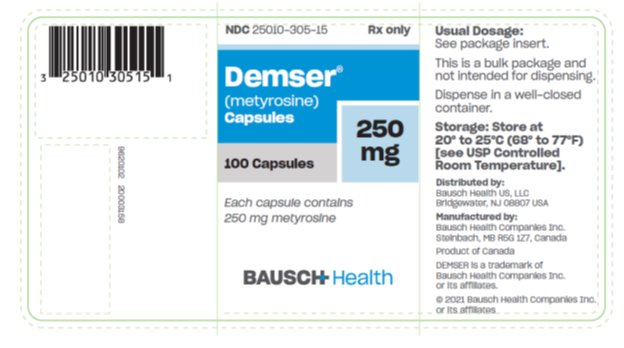
DEMSER is supplied as capsules for oral administration. Each capsule contains 250 mg metyrosine.
Inactive ingredients are colloidal silicon dioxide, gelatin, hydroxypropyl cellulose, magnesium stearate, titanium dioxide, FD&C Blue 2 and edible black ink.
-
CLINICAL PHARMACOLOGY
DEMSER inhibits tyrosine hydroxylase, which catalyzes the first transformation in catecholamine biosynthesis, i.e., the conversion of tyrosine to dihydroxyphenylalanine (DOPA). Because the first step is also the rate-limiting step, blockade of tyrosine hydroxylase activity results in decreased endogenous levels of catecholamines, usually measured as decreased urinary excretion of catecholamines and their metabolites.
In patients with pheochromocytoma, who produce excessive amounts of norepinephrine and epinephrine, administration of 1 to 4 grams of DEMSER per day has reduced catecholamine biosynthesis from about 35% to 80% as measured by the total excretion of catecholamines and their metabolites (metanephrine and vanillylmandelic acid). The maximum biochemical effect usually occurs within two to three days, and the urinary concentration of catecholamines and their metabolites usually returns to pretreatment levels within three to four days after DEMSER is discontinued. In some patients the total excretion of catecholamines and catecholamine metabolites may be lowered to normal or near normal levels (less than 10 mg/24 hours). In most patients, the duration of treatment has been two to eight weeks, but several patients have received DEMSER for periods of 1 to 10 years.
Most patients with pheochromocytoma treated with DEMSER experience decreased frequency and severity of hypertensive attacks with their associated headache, nausea, sweating, and tachycardia. In patients who respond, blood pressure decreases progressively during the first two days of therapy with DEMSER; after withdrawal, blood pressure usually increases gradually to pretreatment values within two to three days.
Metyrosine is well absorbed from the gastrointestinal tract. From 53% to 88% (mean 69%) was recovered in the urine as unchanged drug following maintenance oral doses of 600 to 4000 mg/24 hours in patients with pheochromocytoma or essential hypertension. Less than 1% of the dose was recovered as catechol metabolites. These metabolites are probably not present in sufficient amounts to contribute to the biochemical effects of metyrosine. The quantities excreted, however, are sufficient to interfere with accurate determination of urinary catecholamines determined by routine techniques.
Plasma half-life of metyrosine determined over an 8-hour period after single oral doses was 3-3.7 hours in three patients.
For further information, refer to: Sjoerdsma A, Engelman K, Waldman TA, Cooperman LH, Hammond WG. Pheochromocytoma: Current Concepts of Diagnosis and Treatment: Combined Clinical Staff Conference at the National Institutes of Health. Ann Intern Med. 1966;65:1302-1326.
-
INDICATIONS AND USAGE
DEMSER is indicated in the treatment of patients with pheochromocytoma for:
1. Preoperative preparation of patients for surgery
2. Management of patients when surgery is contraindicated
3. Chronic treatment of patients with malignant pheochromocytoma
DEMSER is not recommended for the control of essential hypertension.
- CONTRAINDICATIONS
-
WARNINGS
Maintain Fluid Volume During and After Surgery
When DEMSER is used preoperatively, alone or especially in combination with alpha-adrenergic blocking drugs, adequate intravascular volume must be maintained intraoperatively (especially after tumor removal) and postoperatively to avoid hypotension and decreased perfusion of vital organs resulting from vasodilatation and expanded volume capacity. Following tumor removal, large volumes of plasma may be needed to maintain blood pressure and central venous pressure within the normal range.In addition, life-threatening arrhythmias may occur during anesthesia and surgery, and may require treatment with a beta-blocker or lidocaine. During surgery, patients should have continuous monitoring of blood pressure and electrocardiogram.
Intraoperative Effects
While the preoperative use of DEMSER in patients with pheochromocytoma is thought to decrease intraoperative problems with blood pressure control, DEMSER does not eliminate the danger of hypertensive crises or arrhythmias during manipulation of the tumor, and the alpha-adrenergic blocking drug, phentolamine, may be needed.Interaction with Alcohol
DEMSER may add to the sedative effects of alcohol and other CNS depressants, e.g., hypnotics, sedatives, and tranquilizers. (See PRECAUTIONS, Information for Patients and Drug Interactions.) -
PRECAUTIONS
General
Metyrosine Crystalluria: Crystalluria and urolithiasis have been found in dogs treated with DEMSER (metyrosine) at doses similar to those used in humans, and crystalluria has also been observed in a few patients. To minimize the risk of crystalluria, patients should be urged to maintain water intake sufficient to achieve a daily urine volume of 2000 mL or more, particularly when doses greater than 2 g per day are given. Routine examination of the urine should be carried out. Metyrosine will crystallize as needles or rods. If metyrosine crystalluria occurs, fluid intake should be increased further. If crystalluria persists, the dosage should be reduced or the drug discontinued.
Relatively Little Data Regarding Long-Term Use: The total human experience with the drug is quite limited and few patients have been studied long term.
Chronic animal studies have not been carried out. Therefore, suitable laboratory tests should be carried out periodically in patients requiring prolonged use of DEMSER and caution should be observed in patients with impaired hepatic or renal function.
Information for Patients
When receiving DEMSER, patients should be warned about engaging in activities requiring mental alertness and motor coordination, such as driving a motor vehicle or operating machinery. DEMSER may have additive sedative effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, and tranquilizers.
Patients should be advised to maintain a liberal fluid intake. (See PRECAUTIONS,General.)
Drug Interactions
Caution should be observed in administering DEMSER to patients receiving phenothiazines or haloperidol because the extrapyramidal effects of these drugs can be expected to be potentiated by inhibition of catecholamine synthesis.
Concurrent use of DEMSER with alcohol or other CNS depressants can increase their sedative effects. (See WARNINGS and PRECAUTIONS,Information for Patients.)
Laboratory Test Interference
Spurious increases in urinary catecholamines may be observed in patients receiving DEMSER due to the presence of metabolites of the drug.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenic studies in animals and studies on mutagenesis and impairment of fertility have not been performed with metyrosine.
Nursing Mothers
It is not known whether DEMSER is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when DEMSER is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 12 years have not been established.
Geriatric Use
Clinical studies of DEMSER did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Central Nervous System
Sedation: The most common adverse reaction to DEMSER is moderate to severe sedation, which has been observed in almost all patients. It occurs at both low and high dosages. Sedative effects begin within the first 24 hours of therapy, are maximal after two to three days, and tend to wane during the next few days.
Sedation usually is not obvious after one week unless the dosage is increased, but at dosages greater than 2000 mg/day some degree of sedation or fatigue may persist.
In most patients who experience sedation, temporary changes in sleep pattern occur following withdrawal of the drug. Changes consist of insomnia that may last for two or three days and feelings of increased alertness and ambition. Even patients who do not experience sedation while on DEMSER may report symptoms of psychic stimulation when the drug is discontinued.
Diarrhea
Diarrhea occurs in about 10% of patients and may be severe. Anti-diarrheal agents may be required if continuation of DEMSER is necessary.
Miscellaneous
Infrequently, slight swelling of the breast, galactorrhea, nasal stuffiness, decreased salivation, dry mouth, headache, nausea, vomiting, abdominal pain, and impotence or failure of ejaculation may occur. Crystalluria (see PRECAUTIONS) and transient dysuria and hematuria have been observed in a few patients. Hematologic disorders (including eosinophilia, anemia, thrombocytopenia, and thrombocytosis), increased SGOT levels, peripheral edema, and hypersensitivity reactions such as urticaria and pharyngeal edema have been reported rarely.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Signs of metyrosine overdosage include those central nervous system effects observed in some patients even at low dosages.
At doses exceeding 2000 mg/day, some degree of sedation or feeling of fatigue may persist. Doses of 2000-4000 mg/day can result in anxiety or agitated depression, neuromuscular effects (including fine tremor of the hands, gross tremor of the trunk, tightening of the jaw with trismus), diarrhea, and decreased salivation with dry mouth.
Reduction of drug dose or cessation of treatment results in the disappearance of these symptoms.
The acute toxicity of metyrosine was 442 mg/kg and 752 mg/kg in the female mouse and rat, respectively.
-
DOSAGE AND ADMINISTRATION
The recommended initial dosage of DEMSER for adults and children 12 years of age and older is 250 mg orally four times daily. This may be increased by 250 mg to 500 mg every day to a maximum of 4 g/day in divided doses. When used for preoperative preparation, the optimally effective dosage of DEMSER should be given for at least five to seven days.
Optimally effective dosages of DEMSER usually are between 2 and 3 g/day, and the dose should be titrated by monitoring clinical symptoms and catecholamine excretion. In patients who are hypertensive, dosage should be titrated to achieve normalization of blood pressure and control of clinical symptoms. In patients who are usually normotensive, dosage should be titrated to the amount that will reduce urinary metanephrines and/or vanillylmandelic acid by 50% or more.
If patients are not adequately controlled by the use of DEMSER, an alpha-adrenergic blocking agent (phenoxybenzamine) should be added.
Use of DEMSER in children under 12 years of age has been limited and a dosage schedule for this age group cannot be given.
-
HOW SUPPLIED
DEMSER Capsules 250 mg are blue opaque capsules imprinted with “ATON/305” on body, and “DEMSER” on cap, filled with white to off-white granular free-flowing powder. They are supplied as follows:
NDC 25010-305-15 bottles of 100
Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in a well-closed container.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Steinbach, MB R5G 1Z7, Canada
DEMSER is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2021 Bausch Health Companies Inc. or its affiliates
Rev. 07/2021 9620202 20003159
- Package/Label Display Panel - 250 mg Capsules Label
-
INGREDIENTS AND APPEARANCE
DEMSER
metyrosine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25010-305 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength metyrosine (UNII: DOQ0J0TPF7) (metyrosine - UNII:DOQ0J0TPF7) metyrosine 250 mg Inactive Ingredients Ingredient Name Strength silicon dioxide (UNII: ETJ7Z6XBU4) FD&C Blue No. 2 (UNII: L06K8R7DQK) magnesium stearate (UNII: 70097M6I30) titanium dioxide (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) Product Characteristics Color BLUE (two-toned blue) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code Aton;305;DEMSER Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25010-305-15 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/03/1979 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017871 10/03/1979 Labeler - Bausch Health US, LLC (831922468) Establishment Name Address ID/FEI Business Operations Bausch Health Companies Inc. 253292734 MANUFACTURE(25010-305) , PACK(25010-305)