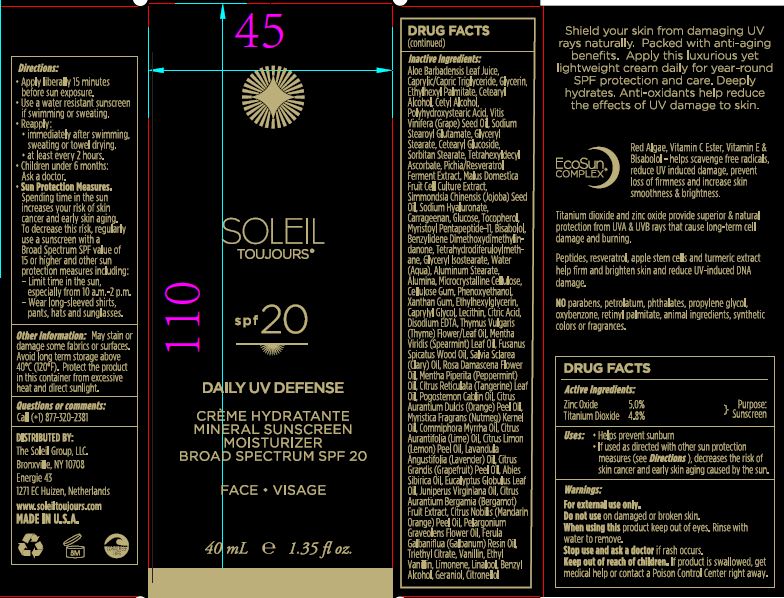
Label: MINERAL SUNSCREEN MOISTURIZER BROAD SPECTRUM SPF 20- zinc oxide and titanium dioxide cream
- NDC Code(s): 62742-4153-1, 62742-4153-2
- Packager: Allure Labs, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 12, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
apply liberally 15 minutes before sun exposure.
use a water resistant sunscreen if swimming or sweating.
reapply:
immidiately aftr swimming, sweating or towel drying.
at least every 2 hours.
children under 6months: ask doctor
Sun protection measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protector measures including:
limit time in the sun, especially from 10:00 am to 2:00 pm.
wear long sleaved shirts, pants, hats and sun glasses.
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Aloe Barbadensis Leaf Juice, Caprylic/Capric Triglyceride, Ethylhexyl Palmitate, Glycerin, Cetearyl Alcohol, Cetyl Alcohol, Polyhydroxysteari Acid, Vitis Vinifera (Grape) Seed Oil, Sodium Stearoyl Glutamate, Glyceryl Stearate, Cetearyl Glucoside, Sorbitan Stearate, Tetrahexyldecyl Ascorbate, Pichia/Resveratrol Ferment Extract, Malus Domestica Fruit Cell Culture Extract, Simmondsia Chinesis (Jojoba) Seed Oil, Sodium Hyaluronate, Carrageenan, Glucose, Tocopherol, Myristoyl Pentapeptide-11, Bisabolol, Benzylidene Dimethoxydimethylindanone, Tetrahydrodiferuloylmethane, Glyceryl Isostearate, Water (Aqua), Aluminum Stearate, Alumina, Microcrystalline Cellulose, Cellulose Gum, Phenoxyethanol, Xanthan Gum, Ethylhexylglycerin, Caprylyl Glycol, Lecithin, Citric Acid, Disodium EDTA, Thymus Vulgaris (Thyme) Flower/Leaf Oil, Mentha Viridis (Spearmint) Leaf Oil, Fusanus Spicatus Wood Oil, Salvia Sclarea (Clary) Oil, Rosa Damascena Flower Oil, Mentha Piperita (Peppermint) Oil, Citrus Reticulata (Tangerine) Leaf Oil, Pogostemon Cablin Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Myristica Fragrans (Nutmeg) Kernel Oil, Commiphora Myrrha Oil, Citrus Aurantifolia (Lime) Oil, Citrus Limon (Lemon) Peel Oil, Lavandula Angustifolia (Lavender) Oil, Citrus Grandis (Grapefruit) Peel Oil, Abies Sibirica Oil, Eucalyptus Globulus Leaf Oil, Juniperus Virginiana Oil, Citrus Aurantium Bergamia (Bergamot) Fruit Extract, Citrus Nobilis (Mandarin Orange) Peel Oil, Pelargonium Graveolens Flower Oil, Ferula Galbaniflua (Galbanum) Resin Oil, Triethyl Citrate, Vanillin, Ethyl Vanillin, Limonene, Linalool, Benzyl Alcohol, Feraniol, Citronellol.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINERAL SUNSCREEN MOISTURIZER BROAD SPECTRUM SPF 20
zinc oxide and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62742-4153 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 50.0 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 48.0 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYL PALMITATE (UNII: 2865993309) CETYL ALCOHOL (UNII: 936JST6JCN) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) VITIS VINIFERA SEED (UNII: C34U15ICXA) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) MALUS DOMESTICA FLOWER (UNII: EF626V855K) SIMMONDSIA CHINENSIS SEED (UNII: D24K2Q1F6H) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CARRAGEENAN (UNII: 5C69YCD2YJ) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-BISABOLOL, (+/-)- (UNII: 36HQN158VC) BENZYLIDENE DIMETHOXYDIMETHYLINDANONE (UNII: 75HIF3C97L) TETRAHYDRODIFERULOYLMETHANE (UNII: 00U0645U03) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) WATER (UNII: 059QF0KO0R) ALUMINUM STEARATE (UNII: U6XF9NP8HM) ALUMINUM OXIDE (UNII: LMI26O6933) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAPRYLYL GLYCOL (UNII: 00YIU5438U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) THYMUS VULGARIS LEAF (UNII: GRX3499643) SPEARMINT OIL (UNII: C3M81465G5) SANTALUM SPICATUM OIL (UNII: H9LVS6REV4) ROSA DAMASCENA FLOWER OIL (UNII: 18920M3T13) PEPPERMINT OIL (UNII: AV092KU4JH) CITRUS RETICULATA LEAF OIL (UNII: 1515UE78IH) PATCHOULI OIL (UNII: F3IN55X5PO) ORANGE PEEL (UNII: TI9T76XD44) NUTMEG OIL (UNII: Z1CLM48948) MYRRH OIL (UNII: H74221J5J4) LIME OIL (UNII: UZH29XGA8G) LEMON OIL (UNII: I9GRO824LL) LAVENDER OIL (UNII: ZBP1YXW0H8) GRAPEFRUIT PEEL (UNII: 3582N05Q44) ABIES SIBIRICA LEAF OIL (UNII: XRY0V4VZKZ) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) JUNIPERUS VIRGINIANA OIL (UNII: PAD4FN7P2G) BERGAMOT OIL (UNII: 39W1PKE3JI) MANDARIN OIL (UNII: NJO720F72R) GALBANUM OIL (UNII: 211UF7M8N1) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) VANILLIN (UNII: CHI530446X) ETHYL VANILLIN (UNII: YC9ST449YJ) LIMONENE OXIDE, (+)- (UNII: 278IM94GXB) LINALOOL, (+)- (UNII: F4VNO44C09) BENZYL ALCOHOL (UNII: LKG8494WBH) GERANIOL (UNII: L837108USY) CITRONELLOL ACETATE, (R)- (UNII: 9X45FJL446) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62742-4153-2 1 in 1 CARTON 12/12/2017 1 NDC:62742-4153-1 40 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 12/12/2017 Labeler - Allure Labs, Inc (926831603) Registrant - Allure Labs, Inc (926831603) Establishment Name Address ID/FEI Business Operations Allure Labs, Inc 926831603 manufacture(62742-4153)