Label: NITROUS OXIDE gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 54416-006-01, 54416-006-02, 54416-006-03, 54416-006-04, view more54416-006-05, 54416-006-06, 54416-006-07, 54416-006-08 - Packager: Nordan Smith Welding Supply
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 9, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
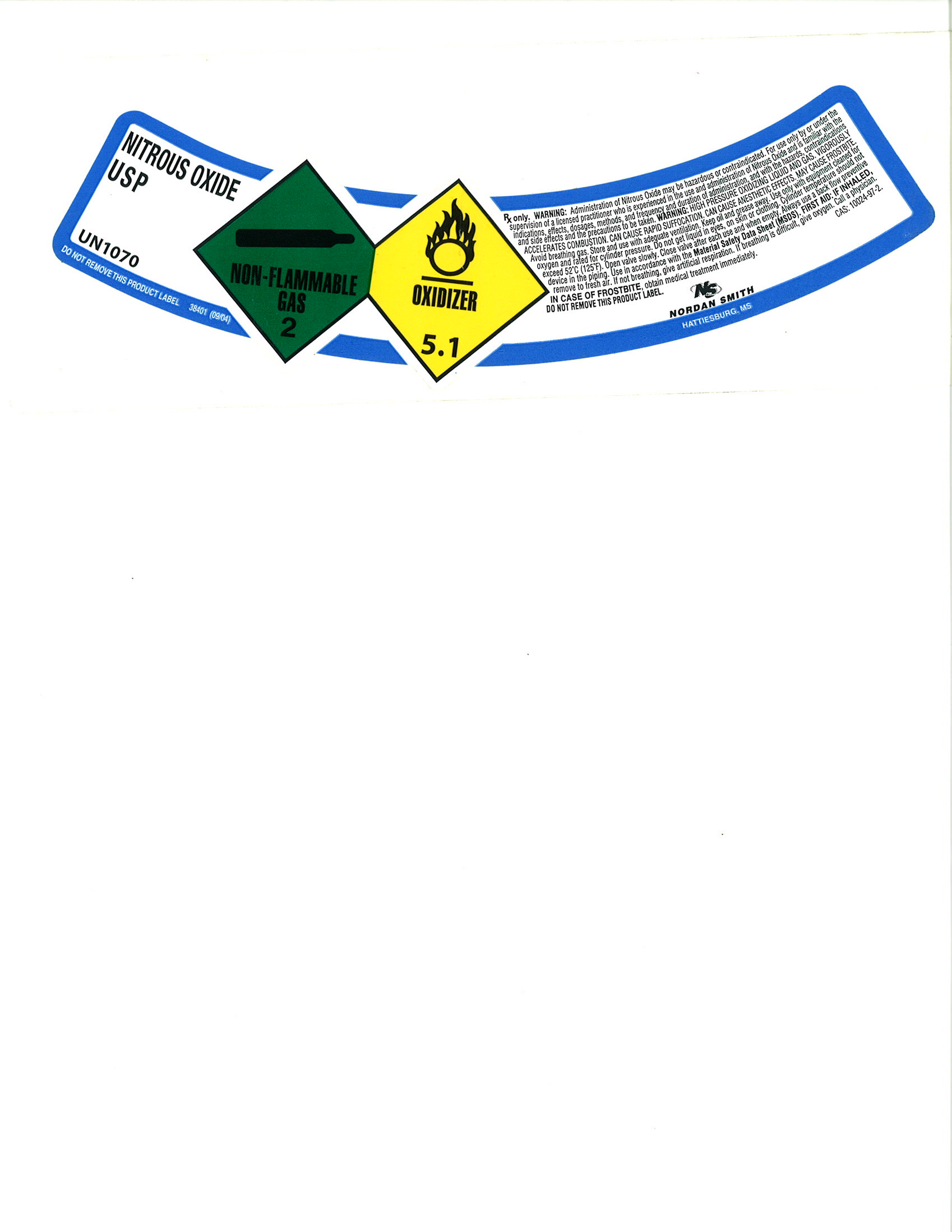
PRINCIPAL DISPLAY PANEL
NITROUS OXIDE USP
UN1070
DO NOT REMOVE THIS PRODUCT LABEL
38401 (09/04)
NON-FLAMMBLE GAS / OXIDIZER
RX only. WARNING: Administration of Nitrous Oxide may be hazardous or contraindicated. For use only by or under the supervision of a licensed practitioner who is experienced in the use and administration of Nitrous Oxide and is familiar with the indications, effects, dosages, methods, and frequency and duration of administration, and with the hazards, contraindications and side effects and the precautions to be taken. WARNING: HIGH PRESSURE OXIDIZING LIQUID AND GAS. VIGOROUSLY ACCELERATES COMBUSTION. CAN CAUSE RAPID SUFFOCATION, CAN CAUSE ANESTHETIC EFFECTS. MAY CAUSE FRPSTBITE. Avoid breathing gas. Store and use with adequate ventilation. Keep oil and grease away. Use only with equipment cleaned for oxygen and rated for cylinder pressure. Do not get liquid in eyes, on skin or clothing. Cylinder temperature should not exceed 52° C (125°F). Open valve slowly. Close valve after each use and when empty. Always use a back flow preventive device in the piping. Use in accordance with the Material Safety Data Sheet (MSDS). FIRST AID: IF INHALED, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Call a physician. IN CASE OF FROSTBITE, obtain medical treatment immediately.
DO NOT REMOVE THIS PRODUCT LABEL.
N/S NORDAN SMITH HATTIESBURG, MS.
CAS: 10024-97-2.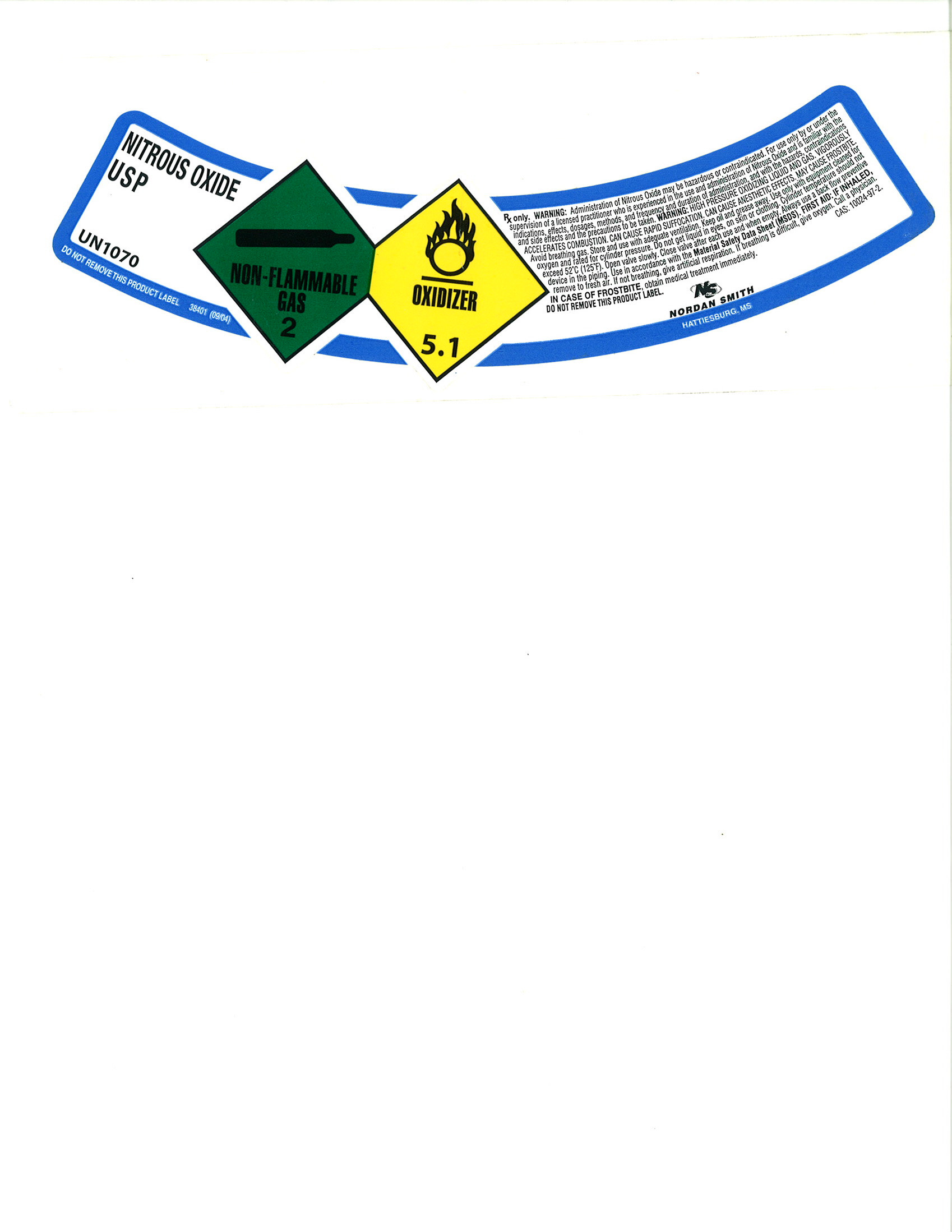
-
INGREDIENTS AND APPEARANCE
NITROUS OXIDE
nitrous oxide gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54416-006 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nitrous Oxide (UNII: K50XQU1029) (Nitrous Oxide - UNII:K50XQU1029) Nitrous Oxide 990 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54416-006-01 1577 L in 1 CYLINDER 2 NDC:54416-006-02 2464 L in 1 CYLINDER 3 NDC:54416-006-03 4927 L in 1 CYLINDER 4 NDC:54416-006-04 9854 L in 1 CYLINDER 5 NDC:54416-006-05 1847 L in 1 CYLINDER 6 NDC:54416-006-06 12318 L in 1 CYLINDER 7 NDC:54416-006-07 190 L in 1 CYLINDER 8 NDC:54416-006-08 1277 L in 1 CYLINDER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved medical gas 01/04/1995 Labeler - Nordan Smith Welding Supply (062456215) Establishment Name Address ID/FEI Business Operations Nordan Smith Welding Supply 062187120 manufacture