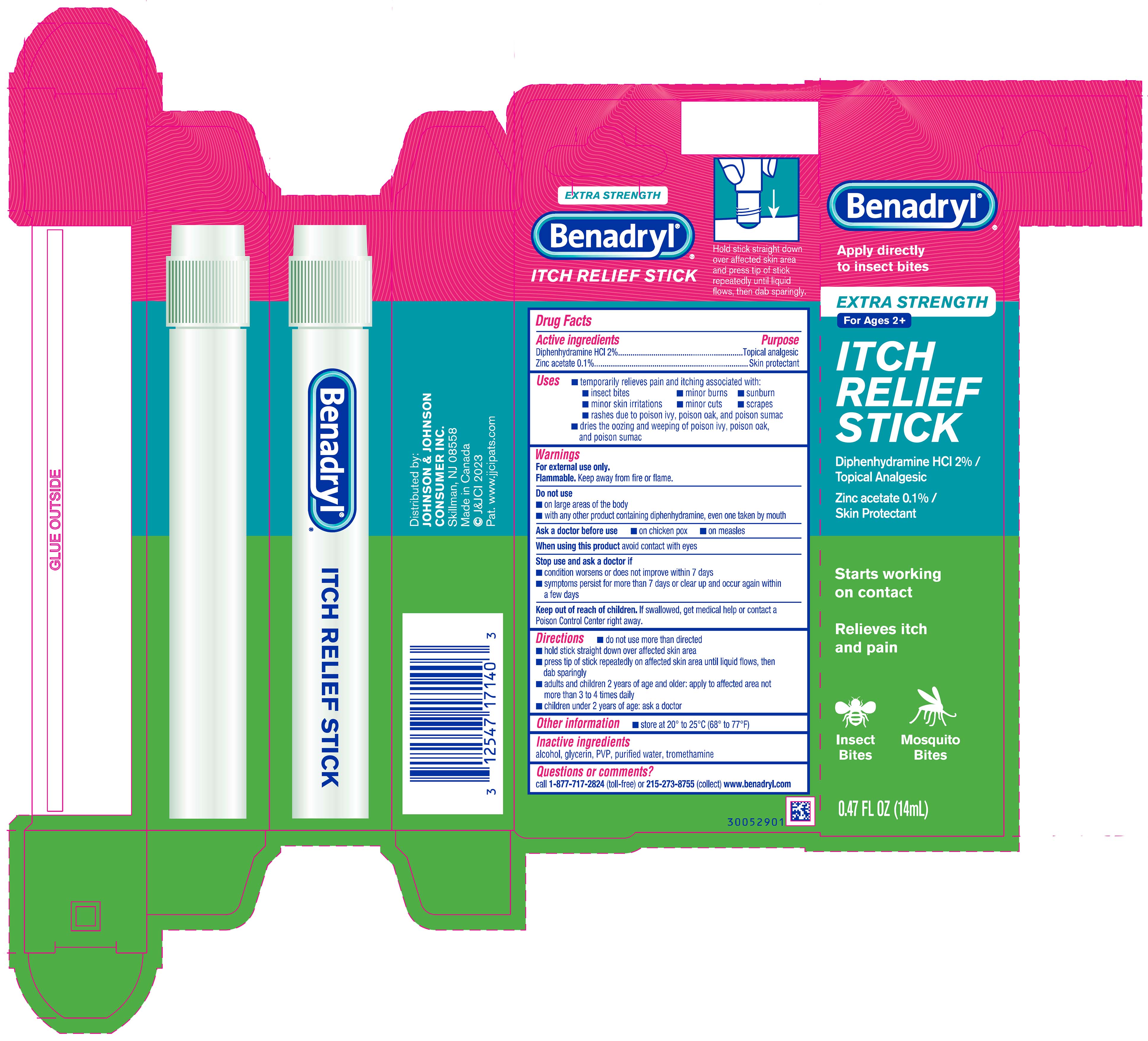
Label: BENADRYL EXTRA STRENGTH ITCH RELIEF- diphenhydramine hydrochloride and zinc acetate solution
- NDC Code(s): 69968-0459-1
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only.
Flammable. Keep away from fire or flame.
Do not use
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
-
Directions
- do not use more than directed
- hold stick straight down over affected skin area
- press tip of stick repeatedly on affected skin area until liquid flows, then dab sparingly
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 14 mL Applicator Carton
-
INGREDIENTS AND APPEARANCE
BENADRYL EXTRA STRENGTH ITCH RELIEF
diphenhydramine hydrochloride and zinc acetate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0459 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC ACETATE (UNII: FM5526K07A) (ZINC CATION - UNII:13S1S8SF37) ZINC ACETATE 1 mg in 1 mL DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) GLYCERIN (UNII: PDC6A3C0OX) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0459-1 1 in 1 CARTON 06/01/2019 1 14 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/2019 Labeler - Johnson & Johnson Consumer Inc. (118772437)