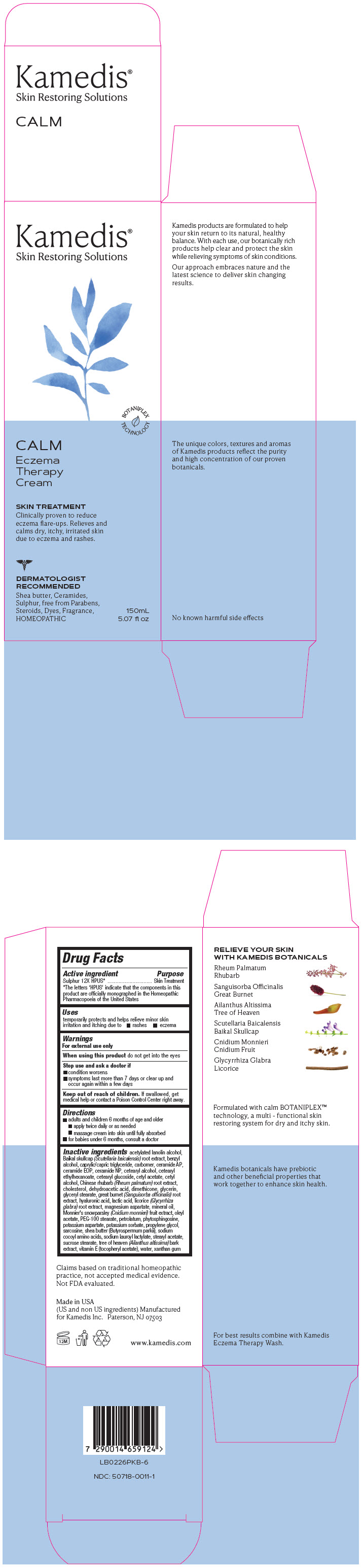
Label: CALM ECZEMA THERAPY CREAM- sulphur 12x cream
- NDC Code(s): 50718-0011-1
- Packager: Kamedis
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active ingredient
Sulphur 12X HPUS*
* The letters 'HPUS' indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United StatesUses
• temporarily protects and helps relieve minor skin irritation and itching due to:
- rashes
- eczema
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away
Directions
- adults and children 6 months of age and older
• apply twice daily or as needed
• massage cream into skin until fully absorbed - for babies under 6 months, consult a doctor
Inactive ingredients
acetylated lanolin alcohol, Baikal skullcap (Scutellaria baicalensis) root extract, benzyl alcohol, caprylic/capric triglyceride, carbomer, ceramide AP, ceramide EOP, ceramide NP, cetearyl alcohol, cetearyl ethylhexanoate, cetearyl glucoside, cetyl acetate, cetyl alcohol, Chinese rhubarb (Rheum palmatum) root extract, cholesterol, dehydroacetic acid, dimethicone, glycerin, glyceryl stearate, great burnet (Sanguisorba officinalis) root extract, hyaluronic acid, lactic acid, licorice (Glycyrrhiza glabra) root extract, magnesium aspartate, mineral oil, Monnier's snowparsley (Cnidium monnieri) fruit extract, oleyl acetate, PEG-100 stearate, petrolatum, phytosphingosine, potassium aspartate, potassium sorbate, propylene glycol, sarcosine, shea butter (Butyrospermum parkii), sodium cocoyl amino acids, sodium lauroyl lactylate, stearyl acetate, sucrose stearate, tree of heaven (Ailanthus altissima) bark extract, vitamin E (tocopheryl acetate), water, xanthan gum
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 150 mL Tube Carton
Kamedis®
Skin Restoring SolutionsBOTANIPLEX
TECHNOLOGYCALM
Eczema
Therapy
CreamSKIN TREATMENT
Clinically proven to reduce
eczema flare-ups. Relieves and
calms dry, itchy, irritated skin
due to eczema and rashes.DERMATOLOGIST
RECOMMENDED
Shea butter, Ceramides,
Sulphur, free from Parabens,
Steroids, Dyes, Fragrance,
HOMEOPATHIC150mL
5.07 fl oz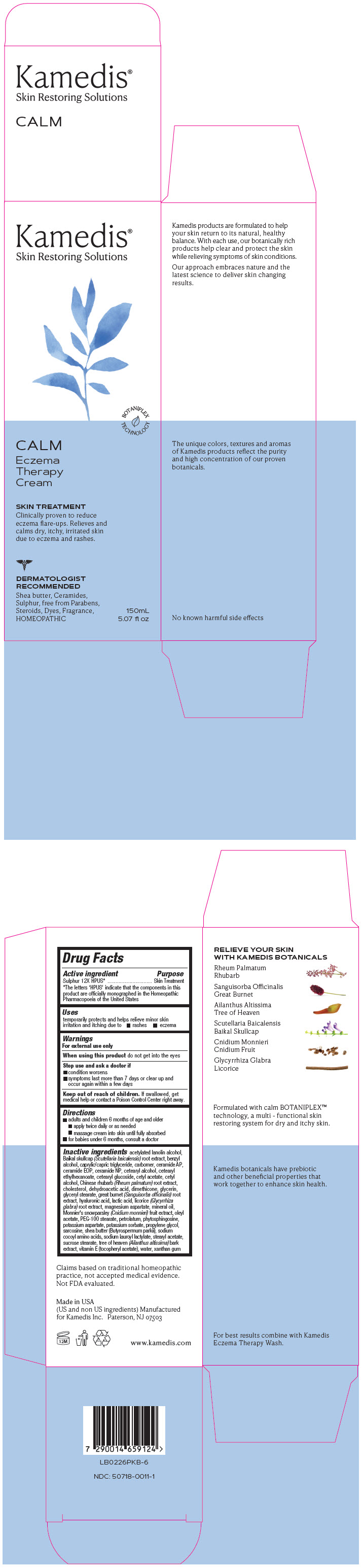
-
INGREDIENTS AND APPEARANCE
CALM ECZEMA THERAPY CREAM
sulphur 12x creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50718-0011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ACETYLATED LANOLIN ALCOHOLS (UNII: SNN716810P) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) BENZYL ALCOHOL (UNII: LKG8494WBH) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CERAMIDE AP (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE NP (UNII: 4370DF050B) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL ETHYLHEXANOATE (UNII: 9M64UO4C25) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CETYL ACETATE (UNII: 4Q43814HXS) CETYL ALCOHOL (UNII: 936JST6JCN) RHEUM PALMATUM ROOT (UNII: G025DAL7CE) CHOLESTEROL (UNII: 97C5T2UQ7J) DEHYDROACETIC ACID (UNII: 2KAG279R6R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) HYALURONIC ACID (UNII: S270N0TRQY) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) MAGNESIUM ASPARTATE (UNII: R17X820ROL) CNIDIUM MONNIERI FRUIT (UNII: V1IA3S3CUS) MINERAL OIL (UNII: T5L8T28FGP) 9-OCTADECENYL ACETATE, (9Z)- (UNII: EU1ETP025C) PEG-100 STEARATE (UNII: YD01N1999R) PETROLATUM (UNII: 4T6H12BN9U) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) POTASSIUM ASPARTATE (UNII: OC4598NZEQ) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SARCOSINE (UNII: Z711V88R5F) SHEA BUTTER (UNII: K49155WL9Y) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) STEARYL ACETATE (UNII: A8005KSX95) SUCROSE STEARATE (UNII: 274KW0O50M) AILANTHUS ALTISSIMA BARK (UNII: 5YM66ALY0U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) sodium cocoyl wheat amino acids (UNII: JW3VT57I11) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50718-0011-1 1 in 1 CARTON 01/01/2018 1 150 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2018 Labeler - Kamedis (080311300) Establishment Name Address ID/FEI Business Operations Biogenesis Inc. 069117328 manufacture(50718-0011)