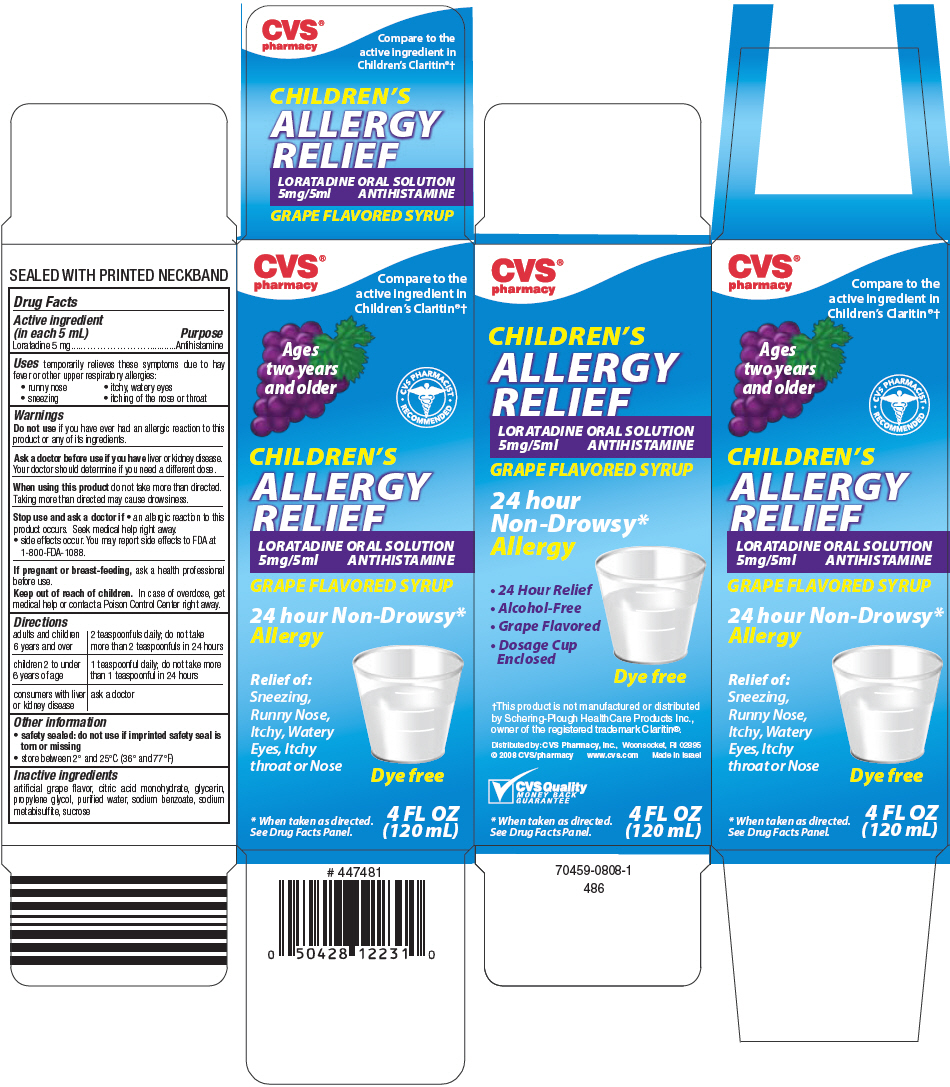
Label: CHILDRENS ALLERGY RELIEF- loratadine solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 59779-446-01, 59779-446-04, 59779-446-08 - Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 27, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Carton
CVS®
pharmacyCompare to the
active ingredient in
Children's Claritin®†Ages
two years
and olderCVS PHARMACIST
RECOMMENDEDCHILDREN'S
ALLERGY
RELIEFLORATADINE ORAL SOLUTION
5mg/5ml ANTIHISTAMINEGRAPE FLAVORED SYRUP
24 hour Non-Drowsy*
AllergyRelief of:
Sneezing,
Runny Nose,
Itchy, Watery
Eyes, Itchy
throat or NoseDye free
* When taken as directed.
See Drug Facts Panel.4 FL OZ
(120 mL)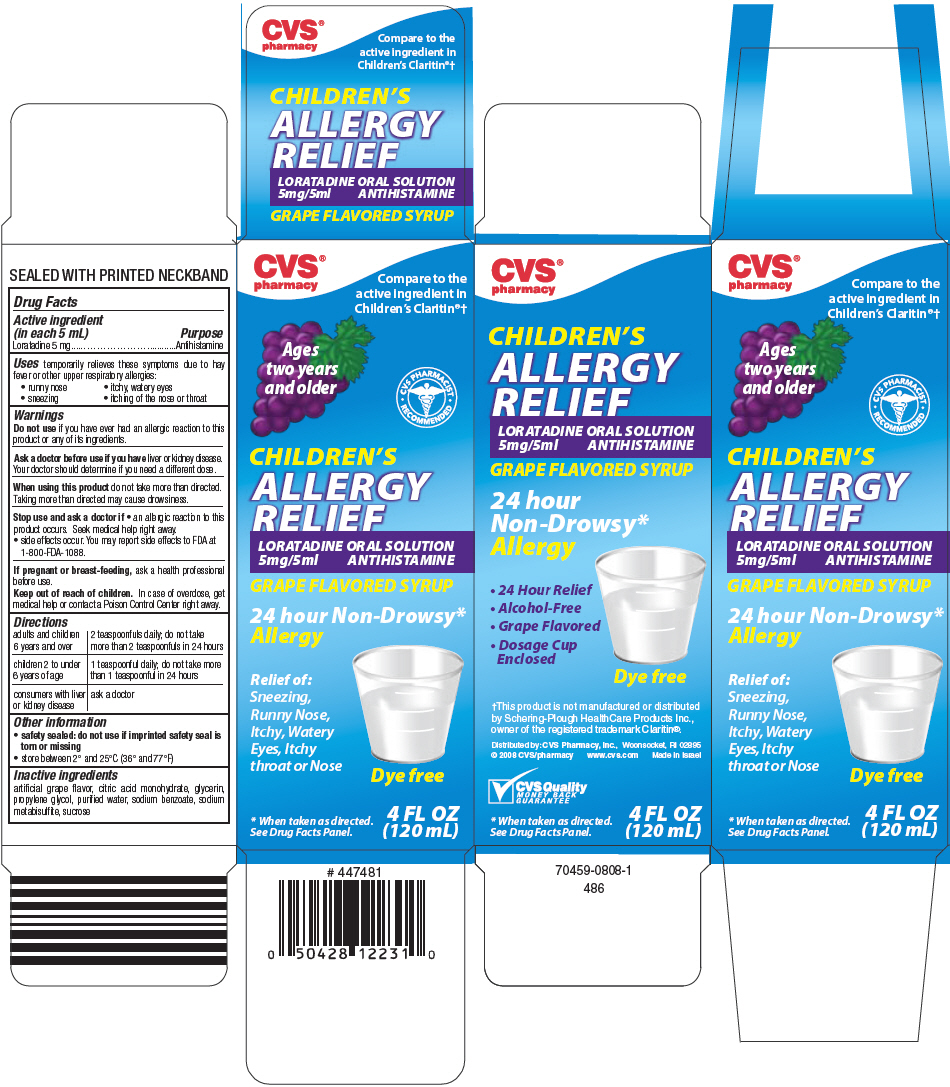
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY RELIEF
loratadine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-446 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Loratadine (UNII: 7AJO3BO7QN) (Loratadine - UNII:7AJO3BO7QN) Loratadine 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength Citric acid monohydrate (UNII: 2968PHW8QP) Glycerin (UNII: PDC6A3C0OX) Propylene glycol (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) Sodium Benzoate (UNII: OJ245FE5EU) Sodium metabisulfite (UNII: 4VON5FNS3C) Sucrose (UNII: C151H8M554) Product Characteristics Color YELLOW (Colorless to slightly yellow) Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-446-04 1 in 1 CARTON 1 60 mL in 1 BOTTLE 2 NDC:59779-446-08 1 in 1 CARTON 2 120 mL in 1 BOTTLE 3 NDC:59779-446-01 1 in 1 CARTON 3 240 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076805 08/20/2004 Labeler - CVS Pharmacy (062312574) Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceutical Indutries Ltd. 600072078 MANUFACTURE(59779-446)