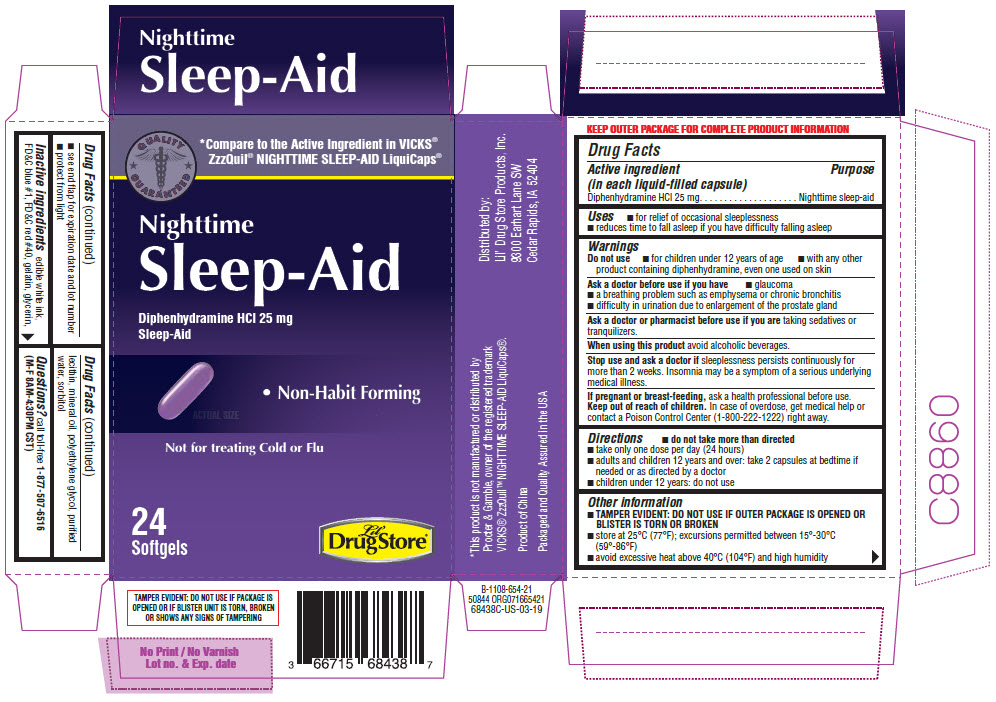
Label: NIGHTTIME SLEEP AID- diphenhydramine hydrochloride capsule, liquid filled
- NDC Code(s): 66715-6743-8
- Packager: Lil' Drug Store Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each liquid-filled capsule)
- Purpose
- Uses
-
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 24 Softgel Blister Pack Carton
-
INGREDIENTS AND APPEARANCE
NIGHTTIME SLEEP AID
diphenhydramine hydrochloride capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-6743 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MINERAL OIL (UNII: T5L8T28FGP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) Product Characteristics Color purple Score no score Shape OVAL Size 15mm Flavor Imprint Code 654 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-6743-8 2 in 1 CARTON 07/15/2019 01/07/2025 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part338 07/15/2019 01/07/2025 Labeler - Lil' Drug Store Products, Inc. (093103646)