Label: BENZO JEL- benzocaine spray, metered
- NDC Code(s): 0404-0018-35
- Packager: Henry Schein Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
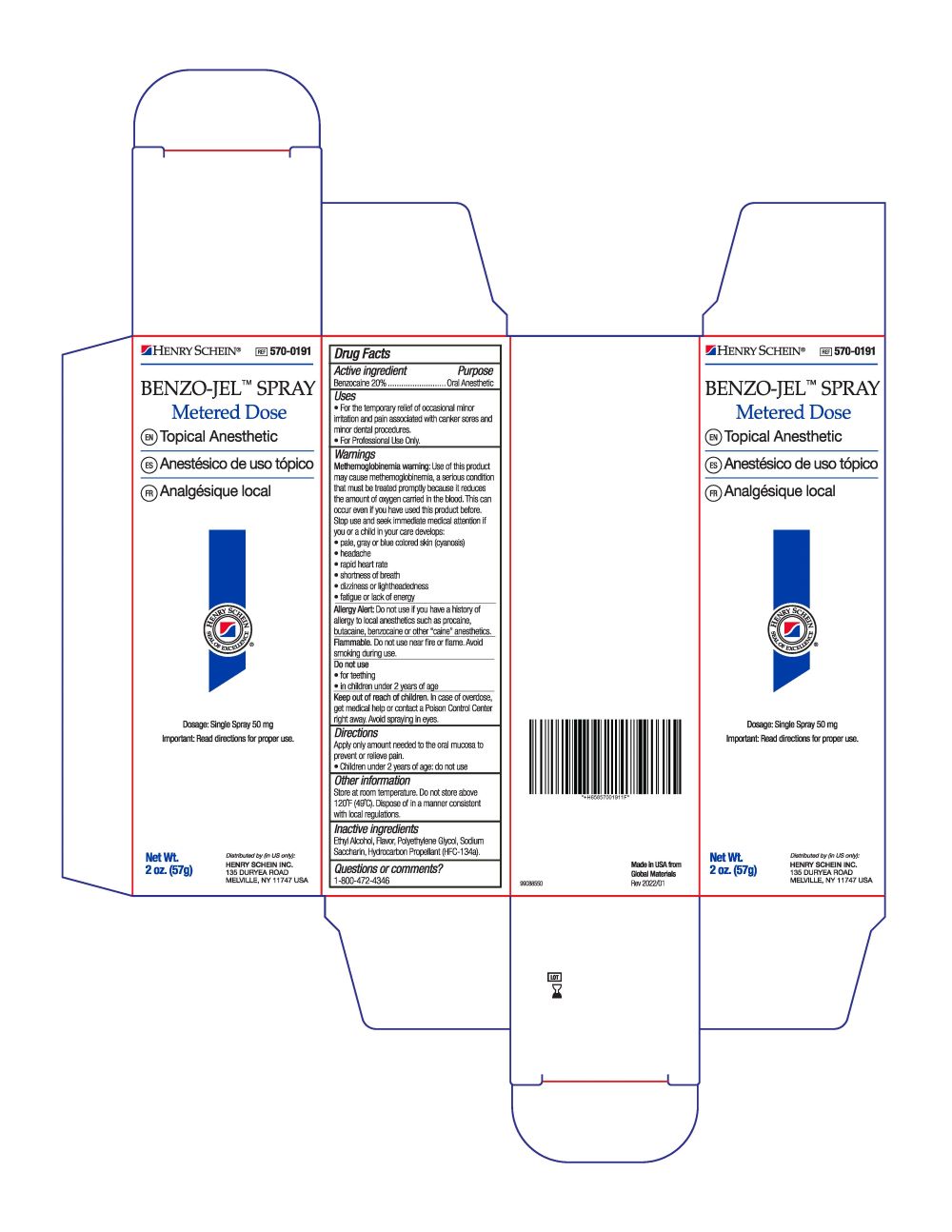
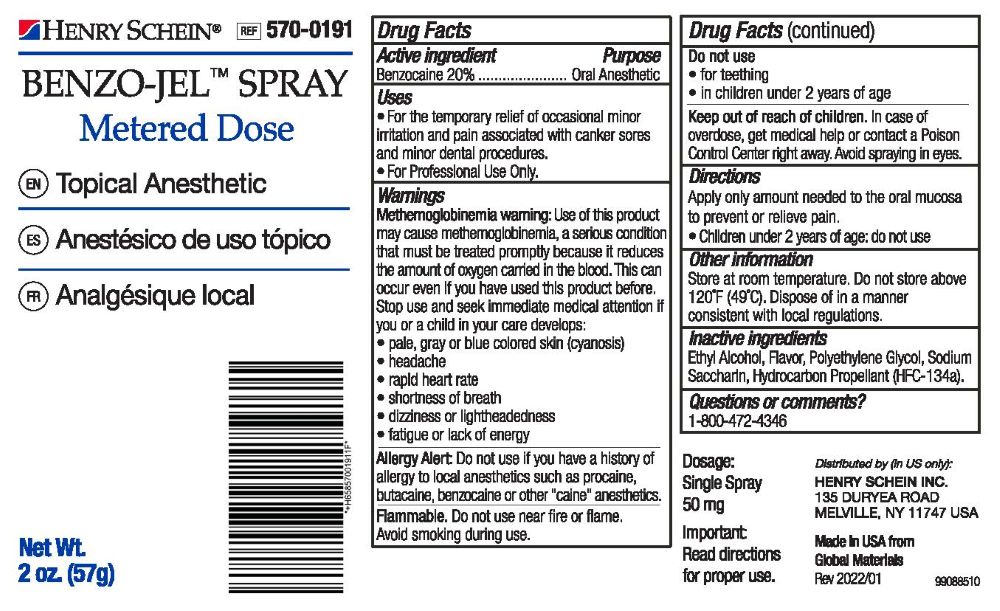
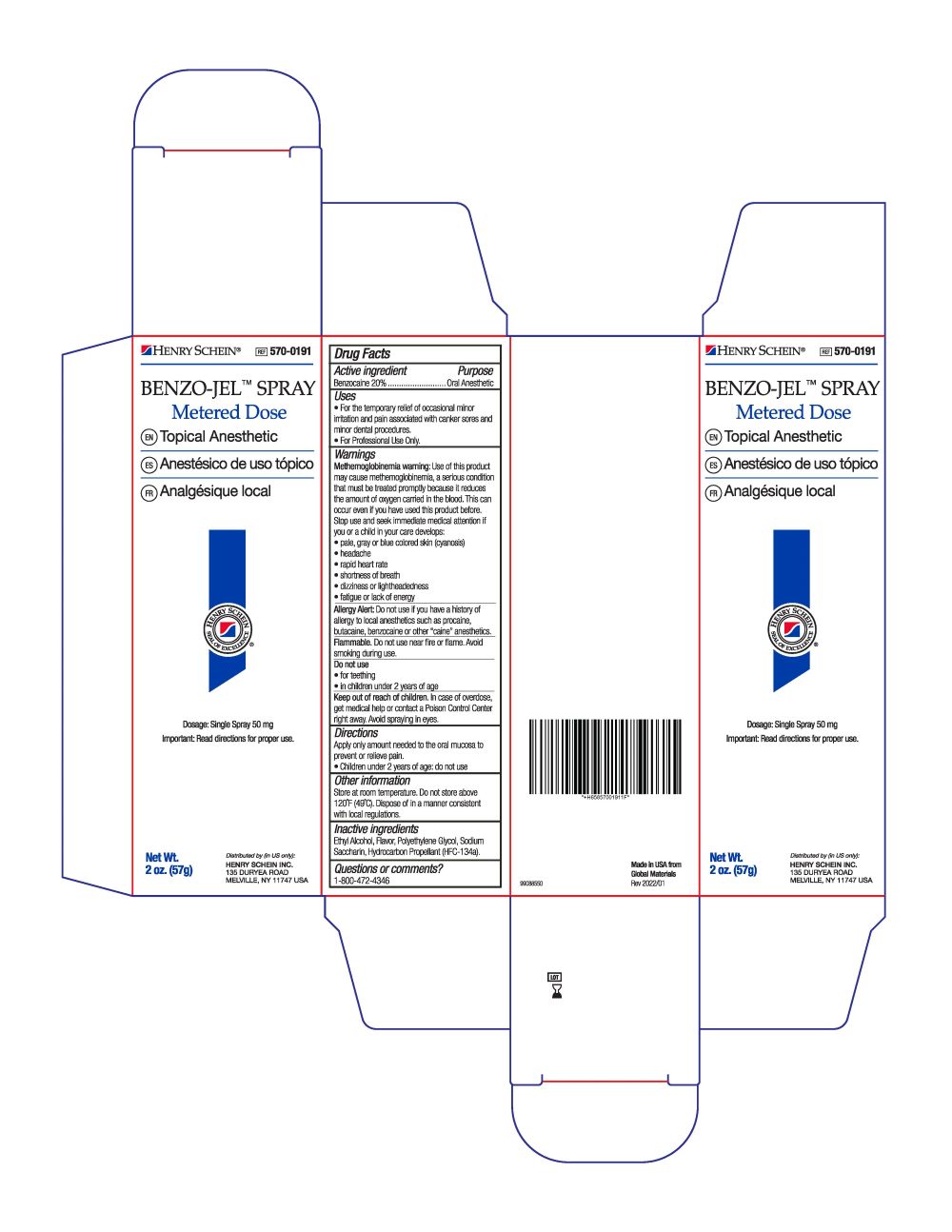
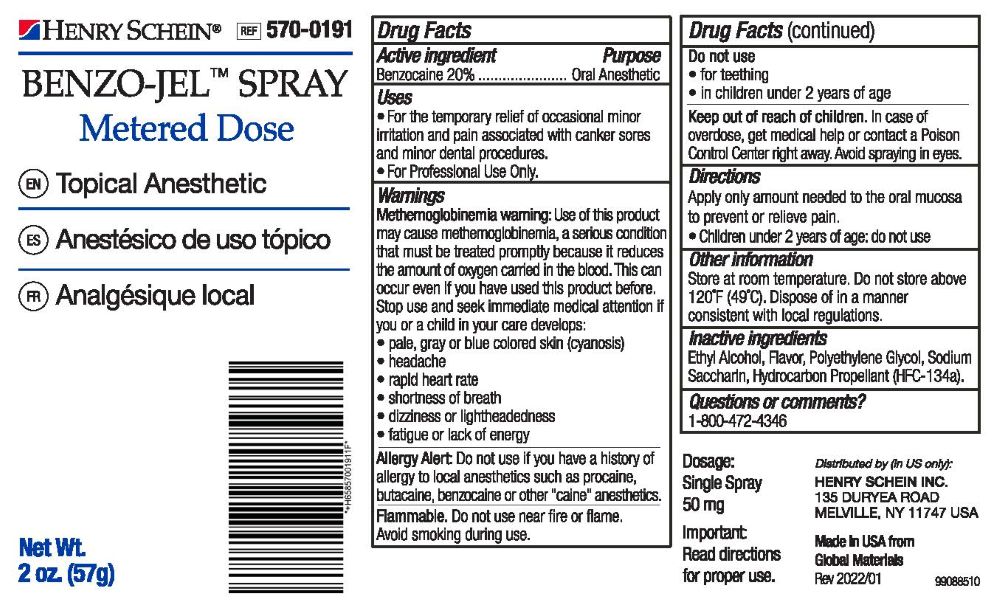
- Active Ingredient
- Purpose
- Uses
-
Warnings
Methemoglobinemia warning: Use of this product may cause methemoglobinemia, a setious condition that must be treated promptiy because it reduces the amount of oxygen canied in the blood. This can occur even if you have used this product before. Slop use and seek immediate medical attention tt you or a child in your care develops:
• pale, gray or blue colored skn (cyanosis)
• headache
• rapid heart rate
• shortress of breath
• dizziness or lightheadedness
• fatigue or lack of energyAllergy Alert: Do not use if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Flammable. Do not use near fire or flame. Avoid smoking during use.
Do not use:
- for teething
- in children under 2 years of age
- Keep out of reach of children.
- Dosage
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BENZO JEL
benzocaine spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0404-0018 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 11.4 g in 57 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color white (CLEAR) Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0404-0018-35 57 g in 1 BOTTLE, SPRAY; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 02/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 02/01/2016 Labeler - Henry Schein Inc. (012430880)