Label: BEERX MUSCLE AND JOINT PAIN RELIEF- capsaicin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 51672-5308-1 - Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only
Do not use ● on wounds, damaged, or irritated skin ● with a heating pad ● if you are allergic to capsicum or chili peppers ● if allergic to bee stings
When using this product ● avoid contact with the eyes or mucous membranes ● do not bandage tightly ● a transient burning sensation may occur upon application but generally disappears in several days ● do not expose the treated area to heat or direct sunlight
Stop use and ask a doctor if ● condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days ● redness is present or irritation develops ● a severe burning sensation occurs
If pregnant or breast-feeding, ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
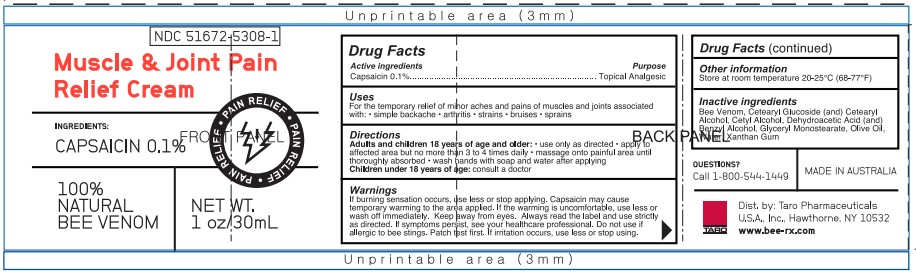
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 51672-5308-1
beeRXTM
Muscle & Joint Pain
Relief Cream
INGREDIENTS:
CAPSAICIN 0.1%
PAIN RELIEF • PAIN RELIEF • PAIN RELIEF
100%
NATURAL
BEE VENOM
NET WT. 1 oz/30 mL
Rapid relief for minor aches and
pains of muscles and joints.
Absorbs quickly - no heavy
residue.
Penetrating pain relief.
MADE IN AUSTRALIA
Questions?
CALL 1-800-544-1449
SAFE AND
NON-TOXIC
NOT TESTED
ON ANIMALS
COLORANT &
FRAGRANCE
FREE
NATURALLY
SOURCED
Dist. by Taro
Pharmaceuticals U.S.A., Inc.
Hawthorne, NY 10532
www.bee-rx.com

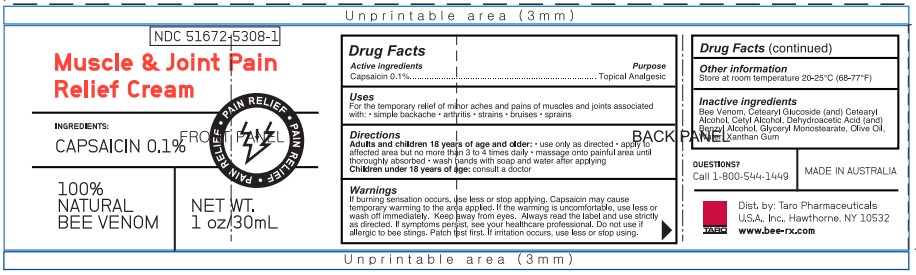
NDC 51672-5308-1
Muscle & Joint Pain
Relief Cream
INGREDIENTS:
CAPSAICIN 0.1%
PAIN RELIEF • PAIN RELIEF • PAIN RELIEF
100%
NATURAL
BEE VENOM
NET WT.
1 oz/30 mL
Dist. by Taro Pharmaceuticals
U.S.A., Inc. Hawthorne, NY 10532
www.bee-rx.com
-
INGREDIENTS AND APPEARANCE
BEERX MUSCLE AND JOINT PAIN RELIEF
capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51672-5308 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.1 g in 30 mL Inactive Ingredients Ingredient Name Strength Cetearyl Glucoside (UNII: 09FUA47KNA) Cetyl Alcohol (UNII: 936JST6JCN) Benzyl Alcohol (UNII: LKG8494WBH) Invert Sugar (UNII: ED959S6ACY) Diglyceryl Monosebacate (UNII: QS4L4WJ1B2) Olive Oil (UNII: 6UYK2W1W1E) Water (UNII: 059QF0KO0R) Xanthan Gum (UNII: TTV12P4NEE) Apis Mellifera Venom (UNII: 76013O881M) Cetostearyl Alcohol (UNII: 2DMT128M1S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51672-5308-1 1 in 1 BOX 10/05/2022 1 30 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 10/05/2022 Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370)