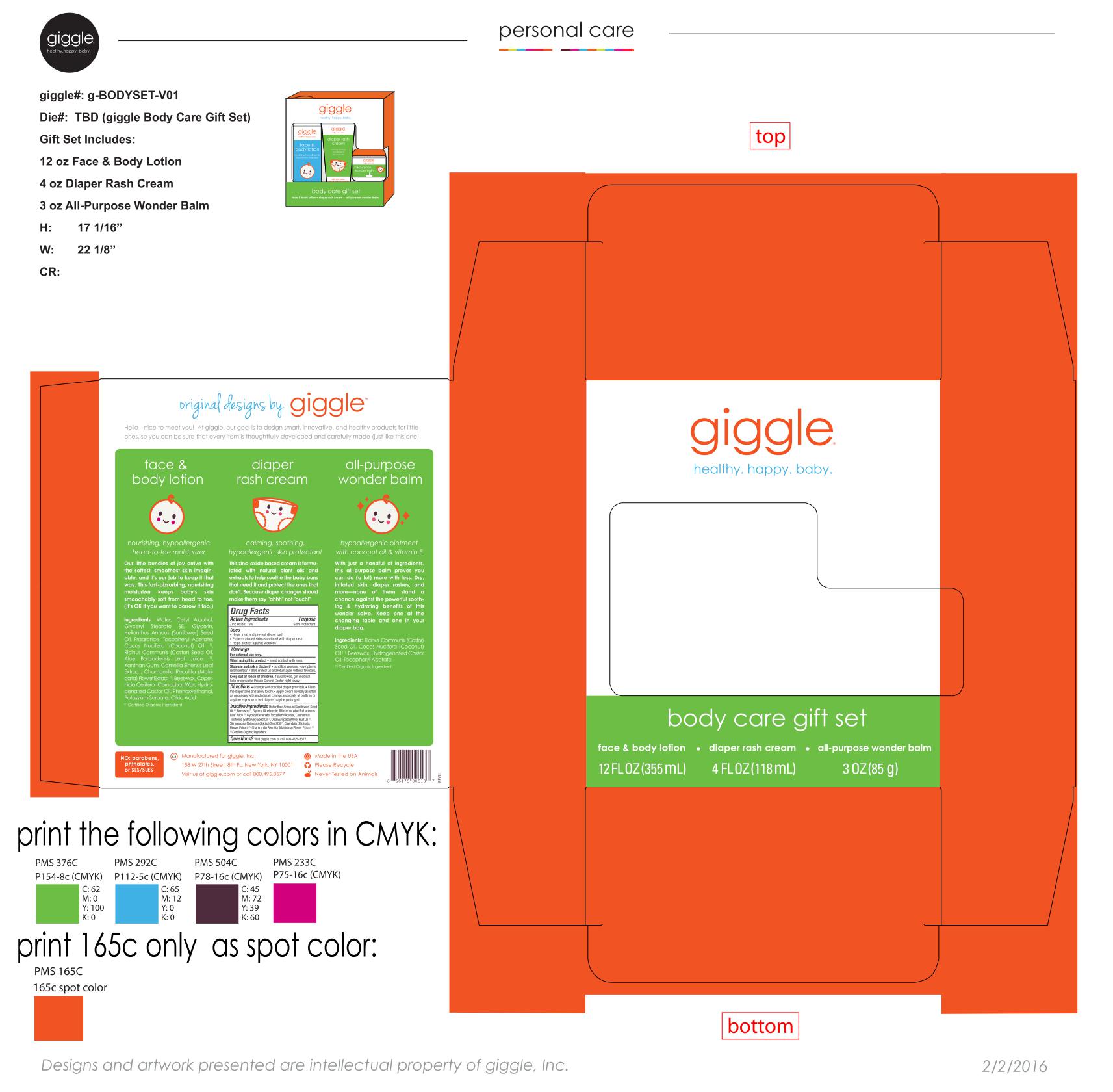
Label: BODY CARE GIFT SET- zinc oxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 70306-1851-4, 70306-9804-9 - Packager: giggle
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 18, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive Ingredients
Helianthus Annuus (Sunflower) Seed Oil (1), Beeswax (1), Glyceryl Dibehenate, Tribehenin, Aloe Barbadensis Leaf Juice (1), Glyceryl Behenate, Tocopheryl Acetate, Carthamus Tinctorius (Safflower) Seed Oil (1), Olea Europae (Olive) Fruite Oil (1), Simmondsia Chinensis (Jojoba) Seed Oil (1), Calendula Officialis Flower Extract (1), Chamomilla Recutita (Matricaria) Flower Extract (1)(1) Certified Organic Ingredient
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BODY CARE GIFT SET
zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70306-9804 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70306-9804-9 1 in 1 KIT 03/01/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 355 mL Part 2 1 JAR 85 g Part 3 1 TUBE 118 mL Part 1 of 3 FACE AND BODY LOTION
lotions, oils, powders, and creams lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR CETYL ALCOHOL (UNII: 936JST6JCN) INGR GLYCERYL STEARATE SE (UNII: FCZ5MH785I) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR SUNFLOWER OIL (UNII: 3W1JG795YI) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR CASTOR OIL (UNII: D5340Y2I9G) INGR GREEN TEA LEAF (UNII: W2ZU1RY8B0) INGR CHAMOMILE (UNII: FGL3685T2X) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR YELLOW WAX (UNII: 2ZA36H0S2V) INGR ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) INGR CARNAUBA WAX (UNII: R12CBM0EIZ) INGR HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Product Characteristics color white C48325 flavor VANILLA C73421 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 355 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 03/01/2016 Part 2 of 3 ALL-PURPOSE WONDER BALM
other baby products ointmentProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR CASTOR OIL (UNII: D5340Y2I9G) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR YELLOW WAX (UNII: 2ZA36H0S2V) INGR HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) INGR ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Product Characteristics color white C48325 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 85 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 03/01/2016 Part 3 of 3 DIAPER RASH CREAM
zinc oxide creamProduct Information Item Code (Source) NDC:70306-1851 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 19 g in 100 mL Inactive Ingredients Ingredient Name Strength CHAMOMILE (UNII: FGL3685T2X) GLYCERYL MONOBEHENATE (UNII: A626UU0W2A) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) JOJOBA OIL (UNII: 724GKU717M) YELLOW WAX (UNII: 2ZA36H0S2V) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) SAFFLOWER OIL (UNII: 65UEH262IS) OLIVE OIL (UNII: 6UYK2W1W1E) ALOE VERA LEAF (UNII: ZY81Z83H0X) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) SUNFLOWER OIL (UNII: 3W1JG795YI) TRIBEHENIN (UNII: 8OC9U7TQZ0) Product Characteristics Color white (Off-White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70306-1851-4 118 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 03/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 03/01/2016 Labeler - giggle (194045402)