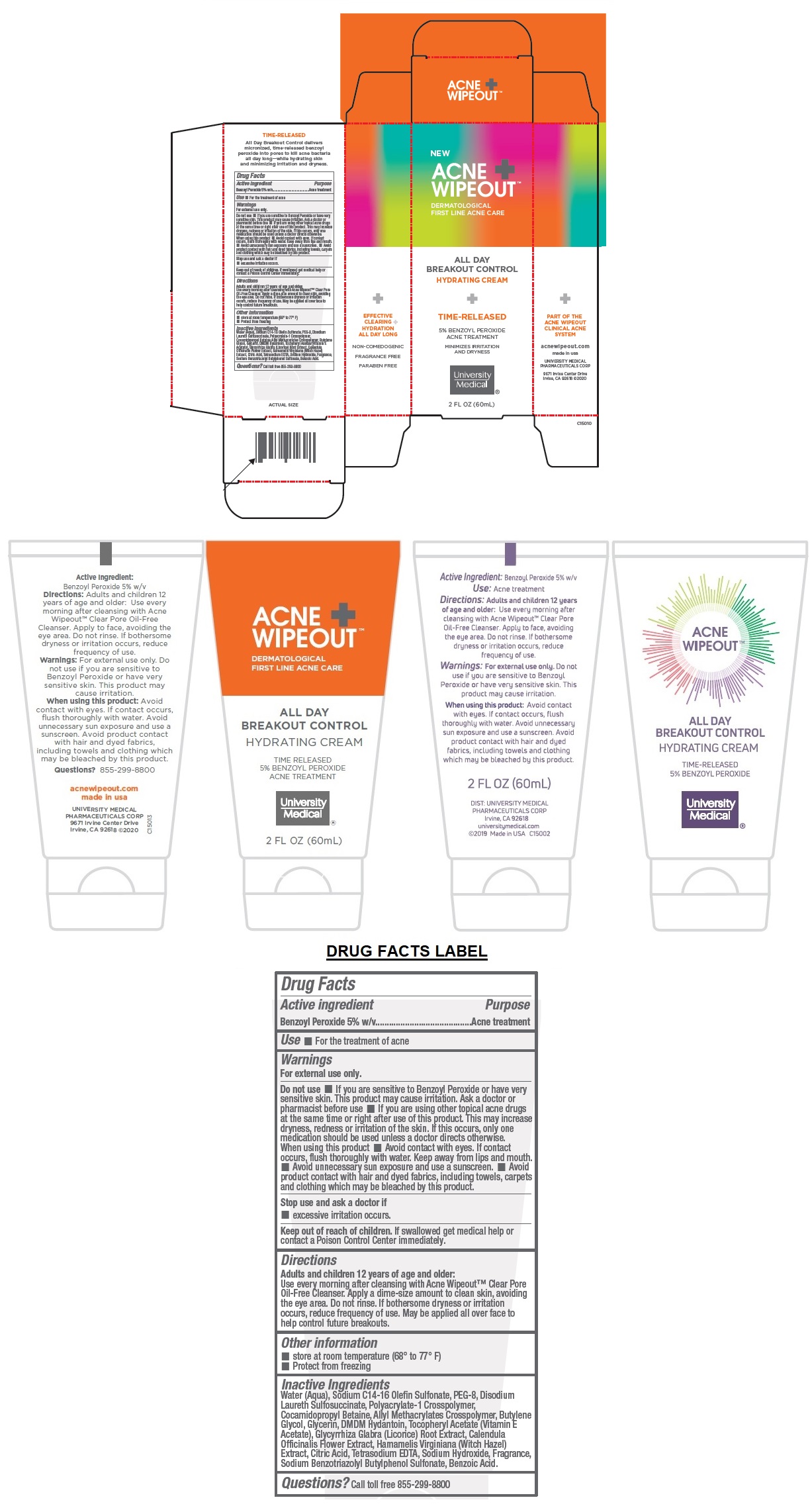
Label: ACNE WIPEOUT ALL DAY BREAKOUT CONTROL- benzoyl peroxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 50544-152-00 - Packager: University Medical Pharmaceuticals Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 2, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only.
Do not use • If you are sensitive to Benzoyl Peroxide or have very sensitive skin. This product may cause irritation. Ask a doctor or pharmacist before use • If you are using other topical acne drugs at the same time or right after use of this product. This may increase dryness, redness or irritation of the skin. If this occurs, only one medication should be used unless a doctor directs otherwise.
When using this product • Avoid contact with eyes. If contact occurs, flush thoroughly with water. Keep away from lips and mouth. • Avoid unnecessary sun exposure and use a sunscreen. • Avoid product contact with hair and dyed fabrics, including towels, carpets and clothing which may be bleached by this product.Stop use and ask a doctor if
• excessive irritation occurs. -
Directions
Adults and children 12 years of age and older:
Use every morning after cleansing with Acne Wipeout™ Clear Pore Oil-Free Cleanser. Apply a dime-size amount to clean skin, avoiding the eye area. Do not rinse. If bothersome dryness or irritation occurs, reduce frequency of use. May be applied all over face to help control future breakouts. - Other information
-
Inactive Ingredients
Water (Aqua), Sodium C14-16 Olefin Sulfonate, PEG-8, Disodium Laureth Sulfosuccinate, Polyacrylate-1 Crosspolymer, Cocamidopropyl Betaine, Allyl Methacrylates Crosspolymer, Butylene Glycol, Glycerin, DMDM Hydantoin, Tocopheryl Acetate (Vitamin E Acetate), Glycyrrhiza Glabra (Licorice) Root Extract, Calendula Officinalis Flower Extract, Hamamelis Virginiana (Witch Hazel) Extract, Citric Acid, Tetrasodium EDTA, Sodium Hydroxide, Fragrance, Sodium Benzotriazolyl Butylphenol Sulfonate, Benzoic Acid.
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
DERMATOLOGICAL FIRST LINE ACNE CARE
HYDRATING CREAM
TIME-RELEASED
5% BENZOYL PEROXIDE ACNE TREATMENT
MINIMIZES IRRITATION AND DRYNESS
PART OF THE ACNE WIPEOUT CLINICAL ACNE SYSTEM
acnewipeout.com
made in usa
UNIVERSITY MEDICAL
PHARMACEUTICALS CORP
9671 Irvine Center Drive
Irvine, CA 92618 ©2020
EFFECTIVE CLEARING + HYDRATION ALL DAY LONGNON-COMEDOGENIC
FRAGRANCE FREE
PARABEN FREE
All Day Breakout Control delivers micronized, time-released benzoyl peroxide into pores to kill acne bacteria all day long—while hydrating skin and minimizing irritation and dryness.
- Packaging
-
INGREDIENTS AND APPEARANCE
ACNE WIPEOUT ALL DAY BREAKOUT CONTROL
benzoyl peroxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50544-152 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) ALLYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: B9J55EA6QX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) DMDM HYDANTOIN (UNII: BYR0546TOW) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM BENZOTRIAZOLYL BUTYLPHENOL SULFONATE (UNII: 0LA2QC9O3Z) BENZOIC ACID (UNII: 8SKN0B0MIM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50544-152-00 1 in 1 CARTON 06/01/2020 1 60 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/01/2020 Labeler - University Medical Pharmaceuticals Corp. (809706252)