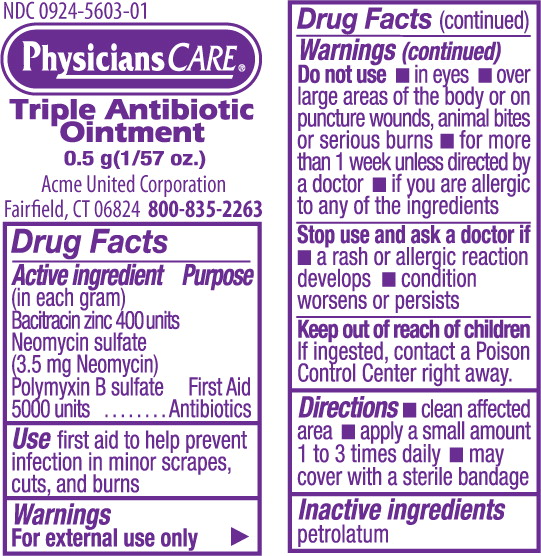
Label: TRIPLE ANTIBIOTIC- bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 0924-5603-01, 0924-5603-04, 0924-5603-05, 0924-5603-06, view more0924-5603-07 - Packager: Acme United Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 28, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses:
-
Warnings:
For external use only. If swallowed, get medical help or contact a Poison Control Center immediately.
STOP USE AND ASK A DOCTOR IF:
- Conditions worsen or clear up and then recur
- The condition persists for more than 7 days
- A rash or allergic reaction occurs
- Directions:
- Other information:
- Inactive ingredients:
- Questions about this product: Call 1-800-835-2263 with any questions about this product.
- PRINCIPAL DISPLAY PANEL – packet
-
PRINCIPAL DISPLAY PANEL – 12 count box
PAC-KIT FIRST AID
UNIT
12-00112 EACH
Triple Antibiotic
Ointment PacketsContains 12 ea. First Aid Antibiotic ointment packets
(min. weight each, 0.5 gm) to help prevent infection in minor cuts,
scrapes and burns. See back panel for Drug Facts listing.Distributed by Acme United Corporation Fairfield, CT 06824 · 800-835-2263
-
INGREDIENTS AND APPEARANCE
TRIPLE ANTIBIOTIC
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0924-5603 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength bacitracin zinc (UNII: 89Y4M234ES) (bacitracin zinc - UNII:89Y4M234ES) bacitracin zinc 400 [iU] in 1 g neomycin sulfate (UNII: 057Y626693) (neomycin - UNII:I16QD7X297) neomycin 3.5 mg in 1 g polymyxin b sulfate (UNII: 19371312D4) (polymyxin b - UNII:J2VZ07J96K) polymyxin b sulfate 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength petrolatum (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0924-5603-04 12 in 1 BOX 1 NDC:0924-5603-01 0.5 g in 1 PACKET 2 NDC:0924-5603-05 25 in 1 BOX 2 NDC:0924-5603-01 0.5 g in 1 PACKET 3 NDC:0924-5603-06 60 in 1 BOX 3 NDC:0924-5603-01 0.5 g in 1 PACKET 4 NDC:0924-5603-07 144 in 1 BOX 4 NDC:0924-5603-01 0.5 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 03/21/2012 Labeler - Acme United Corporation (001180207) Registrant - Safetec of America, Inc. (874965262) Establishment Name Address ID/FEI Business Operations Safetec of America, Inc. 874965262 MANUFACTURE(0924-5603)