Label: SPECTOGARD- spectinomycin sulfate injection, solution
- NDC Code(s): 61133-4014-1
- Packager: Bimeda, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated June 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
DESCRIPTION
SpectoGard Sterile Solution contains the sulfate salt of spectinomycin, an aminocyclitol antibiotic produced by Streptomyces spectabilis.
Each mL of SpectoGard Sterile Solution contains spectinomycin sulfate tetrahydrate equivalent to 100 mg spectinomycin; and 9.45 mg benzyl alcohol, added as preservative. The pH was adjusted with hydrochloric acid or sodium hydroxide.
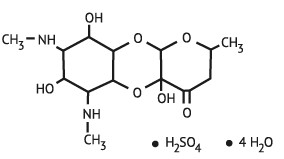
Figure 1. Chemical structure of spectinomycin sulfate tetrahydrate
The chemical name of spectinomycin sulfate tetrahydrate is: Decahydro-4a,7,9-trihydroxy-2-methyl-6,8-bis (methylamino)-4H-pyrano [2,3-b] [1,4] benzodioxin-4-one sulfate, tetrahydrate.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Microbiology
Spectinomycin is bacteriostatic and exerts its antibacterial effect by binding to the 30S ribosome and inhibiting bacterial protein synthesis.
Spectinomycin has activity against a variety of gram-negative bacteria, some mycoplasma, and a limited number of gram-positive bacteria. Generally, it is not active against anaerobic bacteria.
Spectinomycin has demonstrated in vitro and in vivo activity against the three major pathogenic bacteria (Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni) associated with bovine respiratory disease (pneumonia).
Spectinomycin has also demonstrated in vitro activity against Actinomyces pyogenes, Mycoplasma bovis, and Mycoplasma dispar. The clinical significance of this in vitro activity in cattle has not been demonstrated.
- INDICATIONS & USAGE
- CONTRAINDICATIONS
-
RESIDUE WARNING
Residue Warnings
Treated cattle must not be slaughtered for 11 days following last treatment. Dosages administered either in excess of the approved maximum dose or by unapproved routes may result in illegal residues in edible tissues.
A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal.
A milk discard period has not been established for this product in lactating dairy cattle. Do not use in female dairy cattle 20 months of age or older. Use of spectinomycin in this class of cattle may cause drug residues in milk. -
WARNINGS
Human Warnings
NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN.
As with other antibiotics, allergic reactions may occur in previously sensitized individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact with skin, eyes, mouth, and clothing. Persons with a known hypersensitivity to spectinomycin should avoid exposure to this product.
In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Seek medical attention if allergic reactions occur.
The safety data sheet (SDS) contains more detailed occupational safety information. To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet, contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
- PRECAUTIONS
- ADVERSE REACTIONS
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
SpectoGard Sterile Solution is to be administered to cattle at a daily dose of 10 to 15 mg spectinomycin per kg of body weight (4.5 to 6.8 mL per 100 lb body weight). Treatment should be administered at 24-hour intervals for 3 to 5 consecutive days. Selection of dose (10 to 15 mg/kg/day) and duration of treatment (3 to 5 days) should be based on an assessment of the severity of disease, pathogen susceptibility, and clinical response. Do not inject more than 50 mL per site.
SpectoGard Sterile Solution is to be administered to cattle by subcutaneous injection in the neck.
-
SPL UNCLASSIFIED SECTION
ANIMAL SAFETY
Cattle: When spectinomycin sulfate sterile solution was administered at 10 times (150 mg/kg/day) the maximum daily recommended therapeutic dose for 5 days, treatment-related effects included increased relative kidney weights in heifers and steers, squamous and transitional epithelial cells in the urine of steers, and decreased urinary pH in steers. Urinalysis was not performed on the heifers in this study. Minimal injection site reactions were also present at 1 day and 4 days post injection.
When spectinomycin sulfate sterile solution was administered at doses of 14, 45, or 75 mg/kg/day (1X, 3X, or 5X the maximum daily recommended therapeutic dose) for 15 days, treatment-related effects included decreased urinary pH and mild swelling at injection sites. At necropsy, labeled injection sites examined at 1 day and 8 days after injection of 30 mL of spectinomycin sulfate sterile solution had dark red, tan, brown, and/or dark brown areas, often with some expansion (thickening) of the subcutis. Only mild discoloration was observed on gross examination of injection sites at 15 days after injection.
When spectinomycin sulfate sterile solution was administered subcutaneously at a dose of 15 mg/kg/day to 152 crossbred beef calves with naturally occurring BRD in clinical field trials, one calf died following the second daily injection. The cause of death following a gross necropsy was reported as an anaphylactic reaction.
-
STORAGE AND HANDLING
STORAGE CONDITIONS
Store at 20° - 25° C (68° - 77° F). Protect from freezing. Use within 28 days of first puncture and puncture a maximum of 11 times with a needle or 5 times with a dosage delivery device. When using a draw-off spike or needle with bore diameter larger than 4.9 mm, discard any product remaining in the vial immediately after use.
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SPECTOGARD
spectinomycin sulfate injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:61133-4014 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SPECTINOMYCIN SULFATE (UNII: BZ0H4TLF9X) (SPECTINOMYCIN - UNII:93AKI1U6QF) SPECTINOMYCIN 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 9.45 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-4014-1 500 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200694 07/01/2023 Labeler - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda-MTC 256232216 manufacture Establishment Name Address ID/FEI Business Operations Zhejiang Jinhua Conba Bio-Pharm Co., Ltd. 654210624 api manufacture


