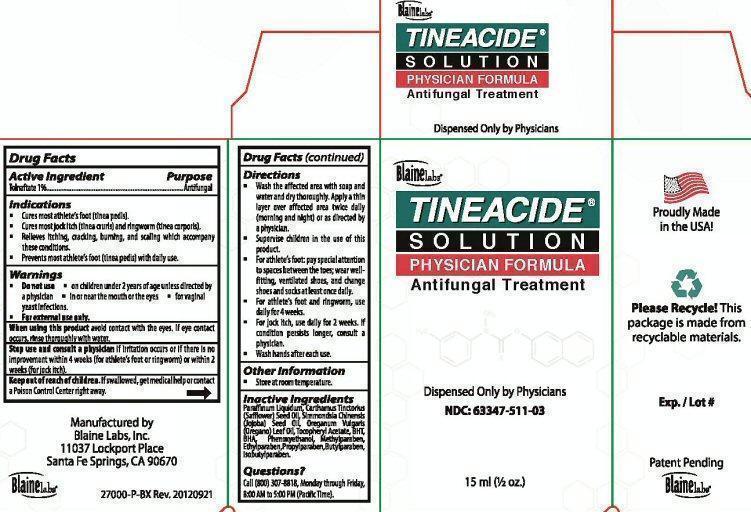
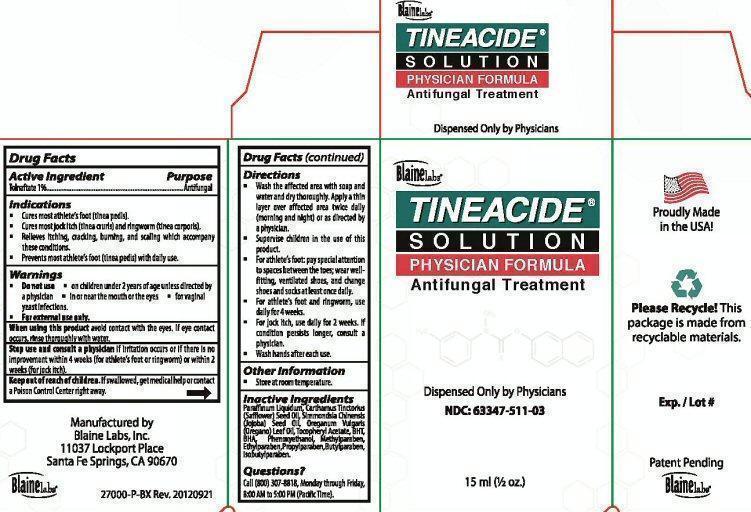
Label: TINEACIDE- tolnaftate liquid
- NDC Code(s): 63347-511-03
- Packager: Blaine Labs Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Keep Out Of Reach Of Children
- Indications
- Warnings
- When Using This Product
- Stop Use And Consult A Physician
-
Directions
Directions
- Wash the affected ares with soap and water and dry thoroughly. Apply a thin layer over affected area twice daily (morning and night) or as directed by a physician.
- Supervise children in the use of this product.
- For athlete's foot pay special attention to spaces between the toes, wear well-fitting, ventilated shoes, and change shoes and socks at least once daily.
- For athlete's foot and ringworm, use daily for 4 weeks.
- For jock itch, use daily for 2 weeks. If condition persists longer, consult a physician.
- wash hands after each use.
- Other Information
- Questions?
- Inactive Ingredients
-
Package Label Tineacide Solution Physician Formula
Blaine Labs
TINEACIDE SOLUTION PHYSICIAN FORMULA
Antifungal Treatment
Dispensed Only by Physicians
NDC: 63347-511-03
15ml (1/2 oz.)
Proudly Made in the USA
Please Recycle! This package is made from recyclable materials.
Exp. / Lot #
Patent Pending
Blaine Labs
Manufactured by
Blaine Labs, Inc
11037 Lockport Place
Sante Fe Springs, CA 90670
27000-P-BX Rev. 20120921
res
-
INGREDIENTS AND APPEARANCE
TINEACIDE
tolnaftate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63347-511 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 140 mg in 15 mL Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) SAFFLOWER OIL (UNII: 65UEH262IS) JOJOBA OIL (UNII: 724GKU717M) OREGANO LEAF OIL (UNII: 7D0CGR40U1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) BUTYLPARABEN (UNII: 3QPI1U3FV8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63347-511-03 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/19/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 09/19/2012 Labeler - Blaine Labs Inc. (017314571) Registrant - Blaine Labs Inc. (017314571) Establishment Name Address ID/FEI Business Operations Blaine Labs Inc. 017314571 manufacture(63347-511)