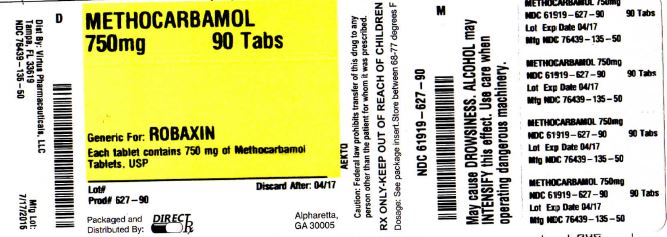
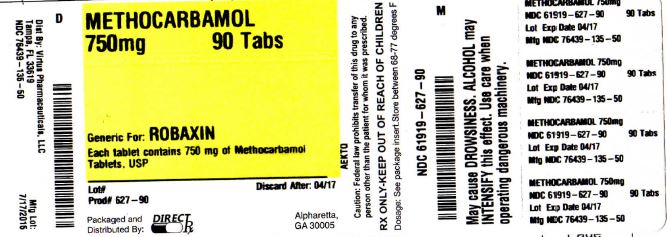
Label: METHOCARBAMOL tablet, film coated
METHOCARBAMOL tablet, coated
METHOCARBAMOL tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 61919-610-60, 61919-627-90, 61919-903-90 - Packager: DirectRX
- This is a repackaged label.
- Source NDC Code(s): 31722-534, 70010-754, 76439-135
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 23, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Description
- Clinical Pharmacology
- Pharmacokinetics
- Elderly
- Special Populations
- Indications and Usage
- Contraindications
- Warnings
- Precautions
- Adverse Reactions
- Overdosage
- Dosage and Administration
- How supplied
- SPL Unclassified
- Package Label
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METHOCARBAMOL
methocarbamol tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61919-627(NDC:76439-135) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOCARBAMOL (UNII: 125OD7737X) (METHOCARBAMOL - UNII:125OD7737X) METHOCARBAMOL 750 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TRIACETIN (UNII: XHX3C3X673) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color yellow Score no score Shape CAPSULE Size 19mm Flavor Imprint Code AP211 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61919-627-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200958 01/01/2015 METHOCARBAMOL
methocarbamol tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61919-610(NDC:70010-754) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOCARBAMOL (UNII: 125OD7737X) (METHOCARBAMOL - UNII:125OD7737X) METHOCARBAMOL 500 mg Inactive Ingredients Ingredient Name Strength POVIDONE (UNII: FZ989GH94E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color orange Score 2 pieces Shape ROUND Size 13mm Flavor Imprint Code G;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61919-610-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 04/18/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209312 04/18/2019 METHOCARBAMOL
methocarbamol tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61919-903(NDC:31722-534) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOCARBAMOL (UNII: 125OD7737X) (METHOCARBAMOL - UNII:125OD7737X) METHOCARBAMOL 750 mg Inactive Ingredients Ingredient Name Strength SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) POVIDONE K90 (UNII: RDH86HJV5Z) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (White to Offwhite) Score no score Shape OVAL (Capsule shaped) Size 19mm Flavor Imprint Code 115;H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61919-903-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/23/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090200 04/23/2019 Labeler - DirectRX (079254320) Establishment Name Address ID/FEI Business Operations DirectRX 079254320 repack(61919-627, 61919-903) , relabel(61919-610)