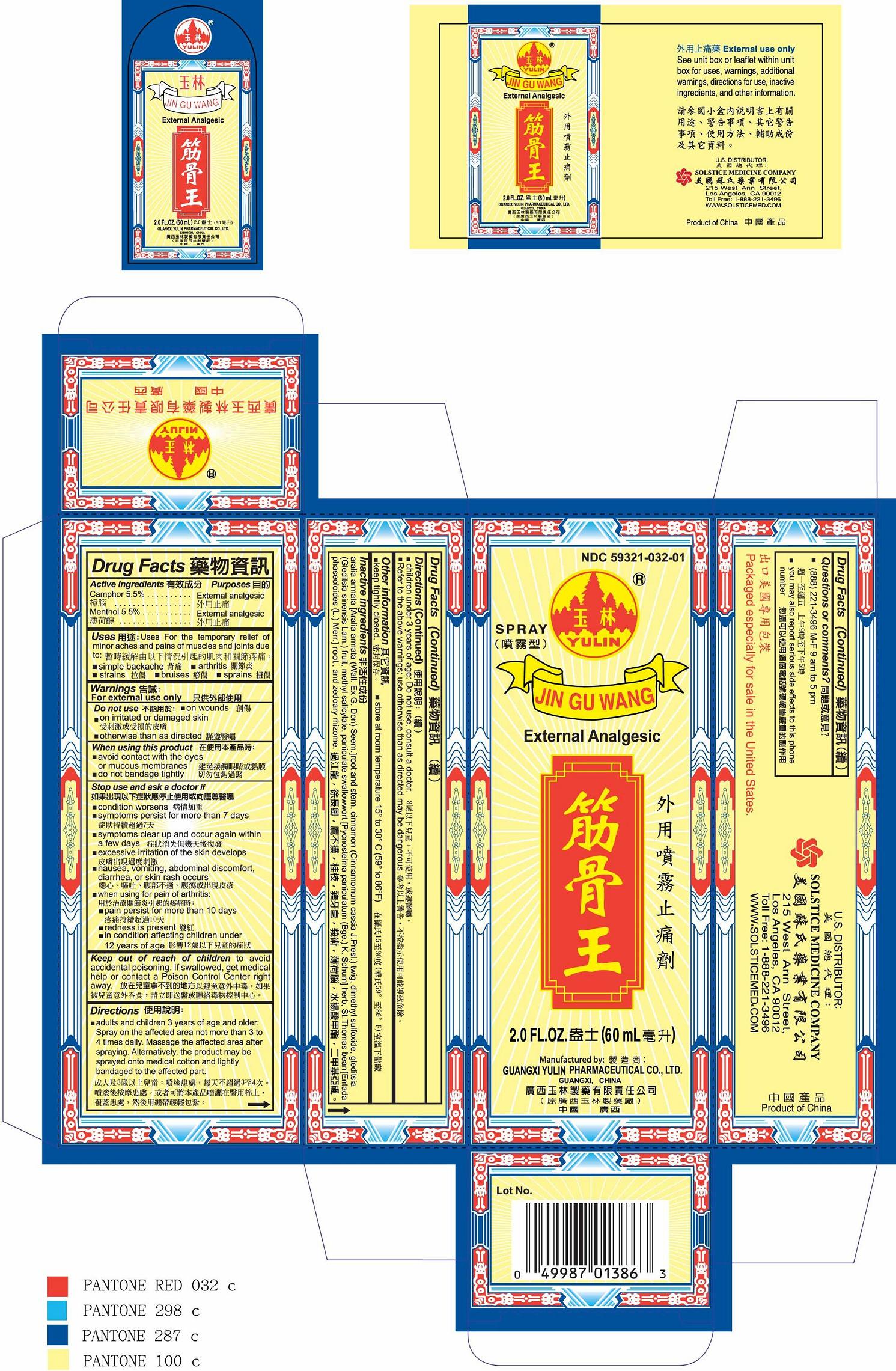
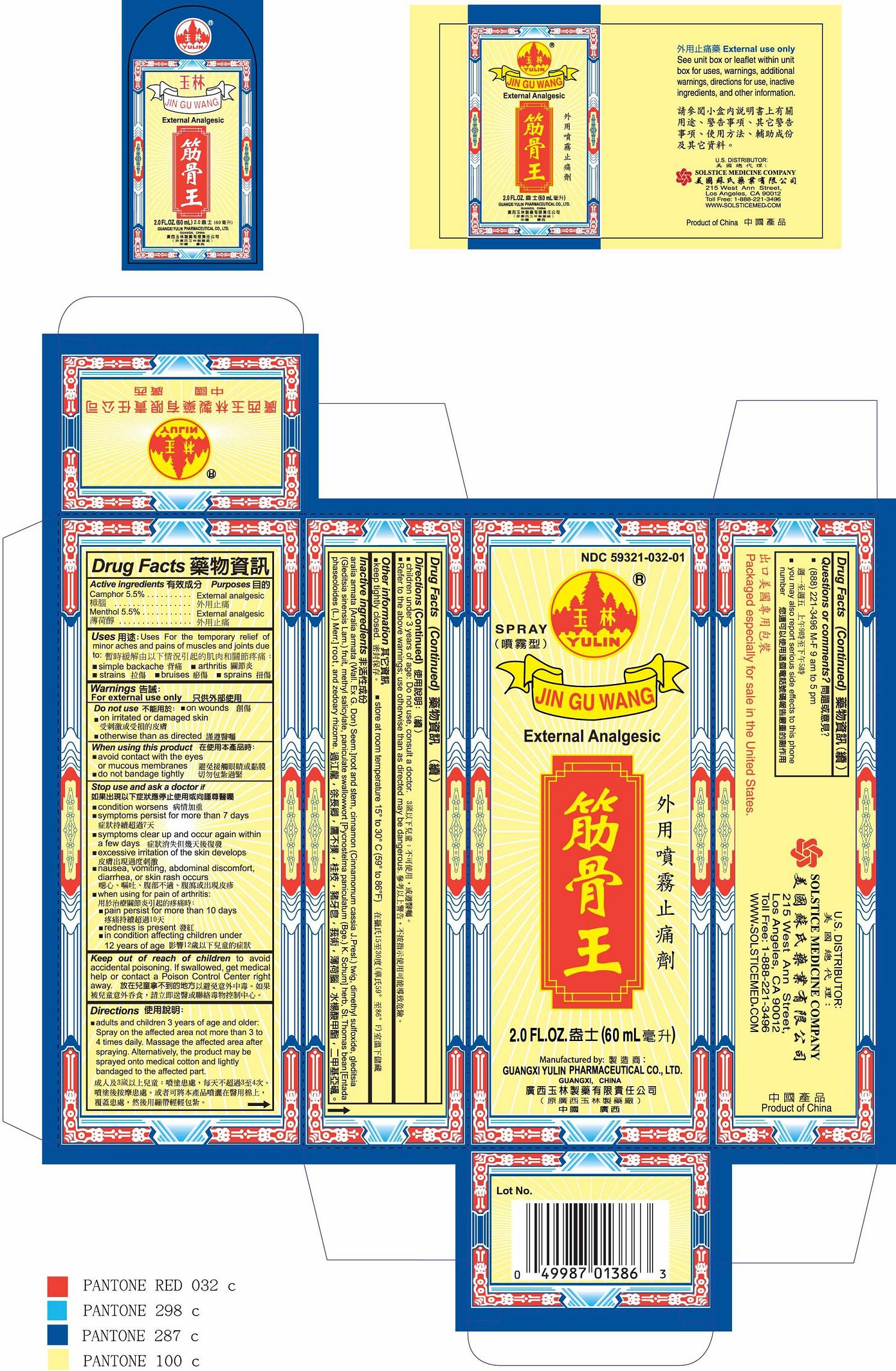
Label: JIN GU WANG- camphor and menthol spray
- NDC Code(s): 59321-032-01
- Packager: GUANGXI YULIN PHARMACEUTICAL CO LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 16, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
condition worsens
symptoms persist for more than 7 days
symptoms clear up and occur again within a few days
excessive irritation of the skin develops
nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
when using for pain of arthritis:
pain persists for more than 10 days
redness is present
in conditions affecting children under 12 years of age
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
adults and children 3 years of age and older: Spray on the affected area not more than 3 to 4 times daily.
Massage the affected area after spraying.
Alternatively, the product may be sprayed onto medical cotton and lightly bandaged to the affected part.
children under 3 years of age: Do not use, consult a doctor
Refer to the above warnings; use otherwise than as directed may be dangerous.
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
aralia armata Aralia armata (Wall. Ex G. Don) Seem root and stem,
cinnamon (Cinnamomum cassia J.Presl.) twig,
dimethyl sulfoxide,
gleditsia (Gleditsia sinensis Lam.) fruit,
methyl salicylate,
paniculate swallowwort Pycnostelma paniculatum (Bge.) K. Schum herb,
St. Thomas bean Entada phaseoloides (L.) Merr. root,
and zedoary rhizome.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JIN GU WANG
camphor and menthol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59321-032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 5.5 g in 100 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5.5 g in 100 mL Inactive Ingredients Ingredient Name Strength CINNAMON (UNII: 5S29HWU6QB) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) GLEDITSIA SINENSIS FRUIT (UNII: A6W0J6UO7Q) METHYL SALICYLATE (UNII: LAV5U5022Y) ENTADA PHASEOLOIDES LEAF (UNII: W20C93AH7K) ZEDOARY (UNII: 123C43G128) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59321-032-01 60 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 04/07/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/07/2011 Labeler - GUANGXI YULIN PHARMACEUTICAL CO LTD (653862581) Establishment Name Address ID/FEI Business Operations GUANGXI YULIN PHARMACEUTICAL CO LTD 653862581 manufacture(59321-032)