Label: EMERGENCY CONTRACEPTIVE- levonorgestrel tablet
- NDC Code(s): 59726-138-01
- Packager: P & L Development, LLC
- This is a repackaged label.
- Source NDC Code(s): 70700-164
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 27, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
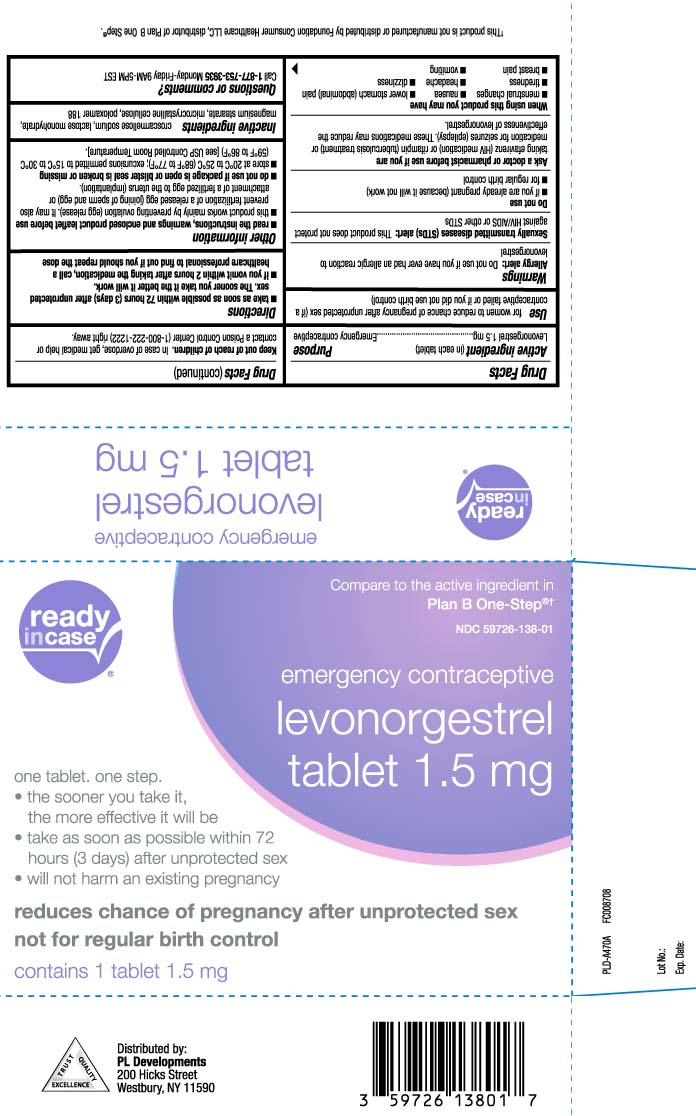
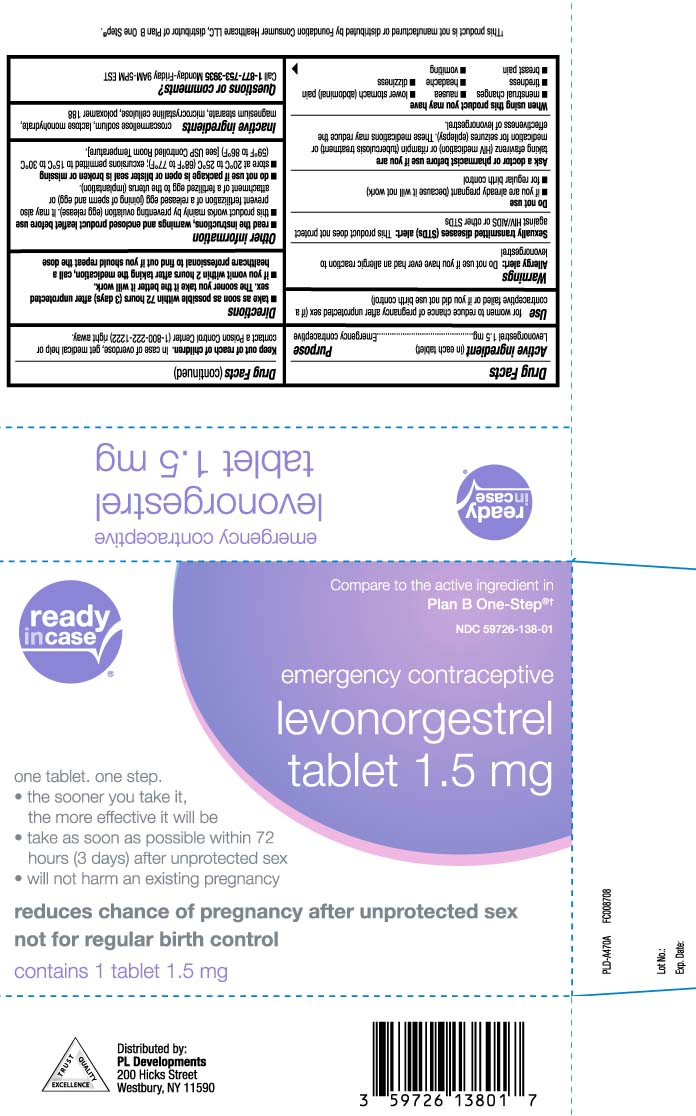
- Active ingredient (in each tablet)
- Purpose
- Use
-
Warnings
Allergy alert: Do not use if you have ever had an allergic reaction to levonorgestrel
Sexually transmitted diseases (STDs) alert: This product does not protect against HIV/AIDS or other STDs - Keep out of reach of children
- Directions
-
Other information
-
read the instructions, warnings and enclosed product leaflet before use
- this product works mainly by preventing ovulation (egg release). It may also prevent fertilization of a release egg (joining of sperm and egg) or attachment of a fertilized egg to the uterus (implantation)
- do not use if package is open or blister seal is broken or missing
- store at 20ºC to 25ºC (68ºF to 77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature].
-
read the instructions, warnings and enclosed product leaflet before use
- Inactive ingredients
- Questions or comments?
-
Principal display panel
Compare to the active ingredient in Plan B One-Step®†
emergency contraceptive
levonorgestrel
Tablet 1.5 mg
one tablet. one step.
- the sooner you take it, the more effective it will be
- take as soon as possible within 72 hours (3 days) after unprotected sex
- will not harm an existing pregnancy
reduces chance of pregnancy after unprotected sex not for regular birth control
tablet 1.5 mg
†This product is not manufactured or distributed by Foundation Consumer Healthcare LLC, distributor of Plan B One Step®.
Distributed by:
PL Developments
200 Hicks Street
Westbury, NY 11590
- Package Label
-
INGREDIENTS AND APPEARANCE
EMERGENCY CONTRACEPTIVE
levonorgestrel tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59726-138(NDC:70700-164) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVONORGESTREL (UNII: 5W7SIA7YZW) (LEVONORGESTREL - UNII:5W7SIA7YZW) LEVONORGESTREL 1.5 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLOXAMER 188 (UNII: LQA7B6G8JG) Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code C;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59726-138-01 1 in 1 CARTON 08/01/2023 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205329 08/01/2023 Labeler - P & L Development, LLC (800014821)