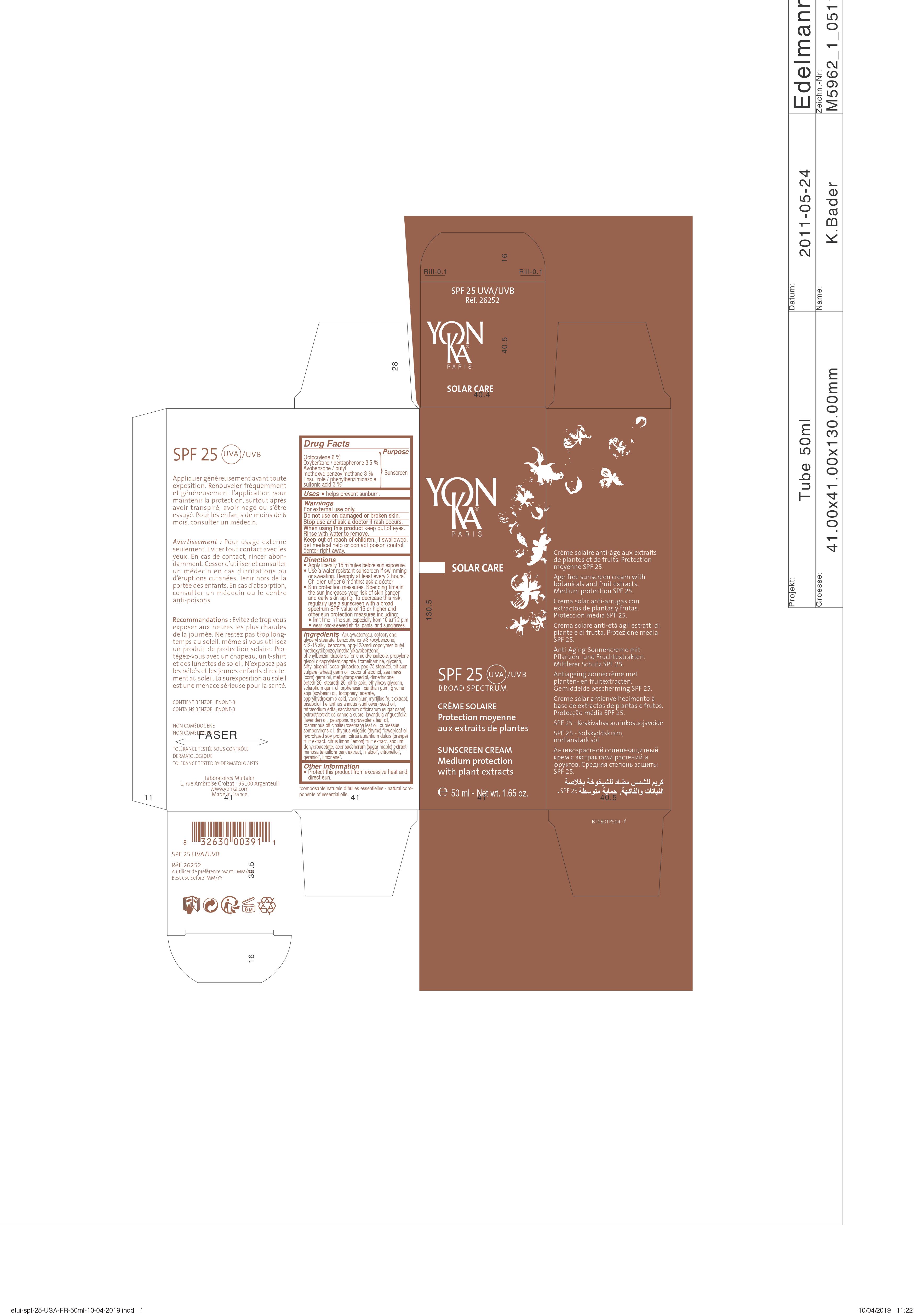
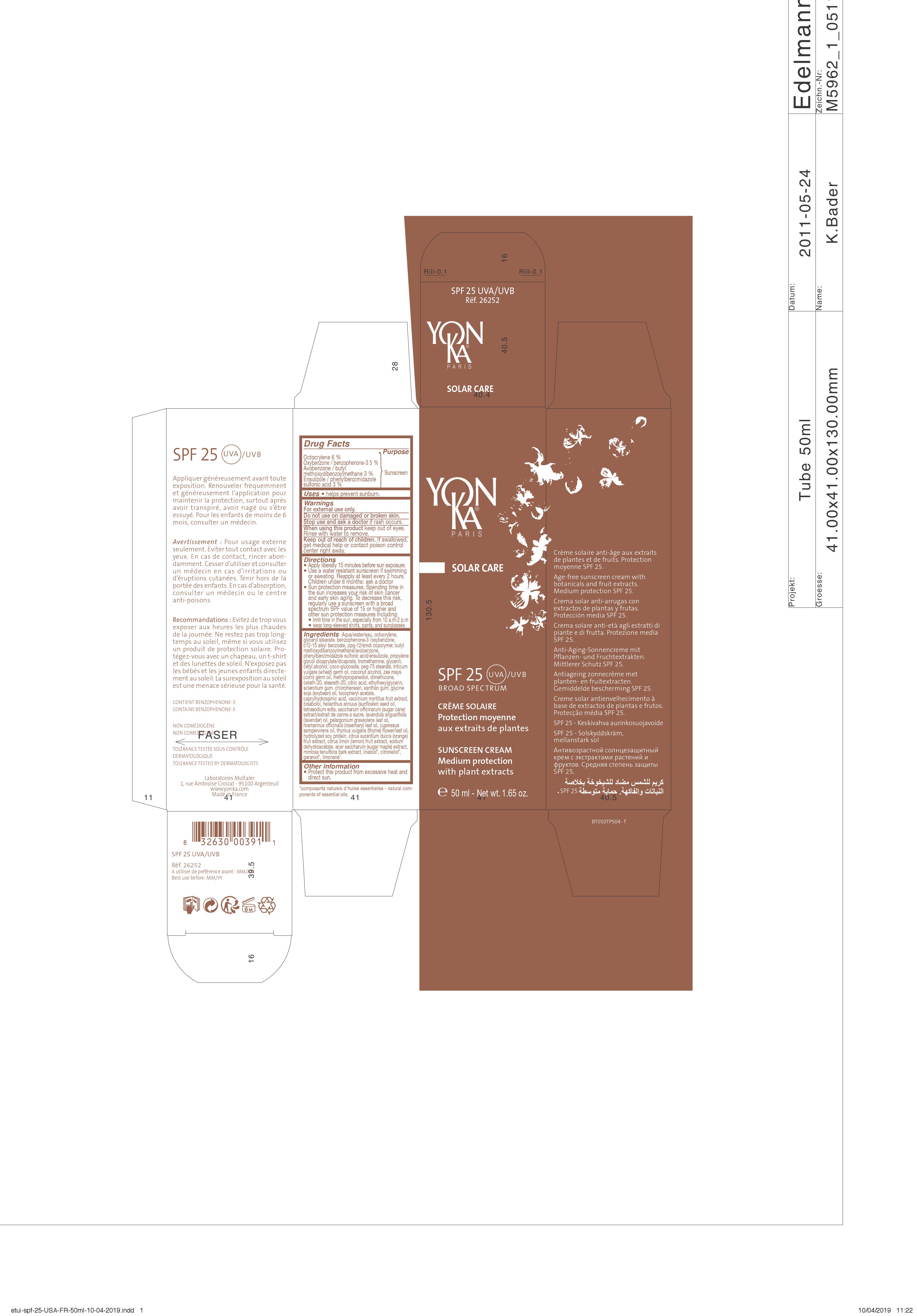
Label: SPF 25 UVA/UVB- ensulizole, oxybenzone, avobenzone, octocrylene cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 51191-3016-1 - Packager: MULTALER ET CIE S.A.S.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 12, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DRUG FACTS
Active ingredients Purpose
Ensulizole 3% {
Oxybenzone 5% { Sunscreen
Avobenzone 3% {
Octocrylene 6% {
SOLAR CARE
SPF 25 UVA/UVB
BROAD SPECTRUM
CREME SOLAIRE
Protection moyenne
aux extraits de plantes
SUNSCREEN CREAM
Medium protection
with plant extracts
Crème solaire anti-âge aux extraits
de plantes et de fruits. Protection
moyenne SPF 25.
Age free sunscreen cream with
botanicals and fruit extracts.
Medium protection SPF 25.
Crema solar anti-arrugas con
extractos de plantas y frutas.
Proteccion media SPF 25.
Anti-aging Sonnencreme mit
Pflanzen- und Fruchtextrakten.
Mittlerer Schutz SPF 25.
Crema solare anti-età agli estratti di
piante e di frutta. Protezione media
SPF 25.
Antiageing Zonnencrème met
planten- en fruitextracten.
Gemiddelde Bescherming SPF 25.
Creme solar antienvelhecimento à
base de extractos de plantas e frutos.
Protecçao média SPF 25.
Appliquer généreusement avant toute
exposition. Renouveler fréquemment
et généreusement l'application pour
maintenir la protection, surtout après
avoir transpiré, avoir nagé ou s'être
essuyé. Pour les enfants de moins de
6 mois, consulter un médecin.
Avertissement : Pour usage externe
seulement. Eviter tout contact avec les
yeux. En cas de contact, rincer abon-
damment. Cesser d'utiliser et consulter
un médecin en cas d'irritations ou
d'éruptions cutanées. Tenir hors de la
portée des enfants. En cas d'absorption,
consulter un médecin ou le centre
anti-poisons.
Recommandations : Eviter de trop vous
exposer aux heures les plus chaudes
de la journée. Ne restez pas trop long-
temps au soleil. même si vous utilisez
un produit de protection solaire. Pro-
tégez-vous avec un chapeau, un t-shirt
et des lunettes de soleil. N'exposez pas
les bébés et les jeunes enfants directe-
ment au soleil. La surexposition au soleil
est une menace sérieuse
pour la santé.
CONTIENT BENZOPHENONE-3
CONTAINS BENZOPHENONE-3
NON COMEDOGENE
NON COMEDOGENIC
TOLERANCE TESTEE SOUS CONTROLE DERMATOLOGIQUE
TOLERANCE TESTED BY DERMATOLOGISTS
Uses • hels prevent sunburn.
Warnings
For external use only.
Do not use on damaged or broken skin.
Stop use and ask a doctor if rash occurs.
When using this product keep out of eyes.
Rinse with water to remove.
Keep out of reach of children. If swallowed,
get medical help or contact poison control
center right away.
Directions
• Apply liberally 15 minutes before sun exposure.
• Use a water resistant sunscreen if swimming or
sweating. Reapply at least every 2 hours.
Children under 6 months : ask a doctor.
• Sun protection measures. Spending time in
the sun increases your risk of skin cancer
and early skin aging. To decrease this risk,
regularly use a sunscreen with a broad
sprectrum SPF value of 15 or higher and
other sun protection measures including:
• limit time in the sun, especially from 10 a.m-2p.m
• wear long-sleeved shirts, pants, and sunglasses.
Other information
• Protect this product from excessive heat and
direct sun.
Inactive ingredients Aqua/water/eau,
alkyl (C12-15) benzoate, glyceryl stearate, ppg-12/smdi
copolymer, propylene glycol dicaprylate/dicaprate,
tromethamine, glycerin, cetyl alcohol, coco-glucoside,
peg-75 stearate, triticum vulgare (wheat) germ oil,
coconut alcohol, zea mays (corn) oil, dimethicone,
ceteth-20, steareth-20, citric acid, sclerotium gum,
xanthan gum, helianthus annuus (sunflower) seed oil,
tocopheryl acetate, vaccinium myrtillus (bilberry) fruit
extract, bisabolol, glycine soja (soybean) oil,
hydrolyzed sweet almond protein, saccharum
officinarum (sugar cane) extract/extrait de canne a
sucre, lavandula angustifolia (lavender) oil, pelargonium
graveolens (geranium) leaf oil, rosmarinus officinalis
(rosemary) leaf oil, cupressus sempervirens (cypress) oil,
thymus vulgaris (thyme) flower/leaf oil, citrus limon fruit
extract / citrus medica limonum (lemon) fruit extract,
citrus aurantium dulcis (orange) fruit extract, hydrolyzed
soy protein, acer saccharum (sugar maple) extract,
mimosa tenuiflora bark extract, sodium hydroxide,
methylpropanediol, ethylhexylglycerin, chlorphenesin,
caprylhydroxamic acid, tetrasodium edta, sodium
dehydroacetate, sodium benzoate, linalool*, citronellol*,
geraniol*, limonene*.
*composants naturels d'huiles essentielles - natural com-
ponents of essentials oils
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SPF 25 UVA/UVB
ensulizole, oxybenzone, avobenzone, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51191-3016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 6 g in 100 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 5 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 3 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERIN (UNII: PDC6A3C0OX) PEG-8/SMDI COPOLYMER (UNII: CCX72L6NY6) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-75 STEARATE (UNII: OT38R0N74H) PROPYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: O4446S9CRA) TROMETHAMINE (UNII: 023C2WHX2V) CETYL ALCOHOL (UNII: 936JST6JCN) COCO GLUCOSIDE (UNII: ICS790225B) .ALPHA.-BISABOLOL, (+/-)- (UNII: 36HQN158VC) CHLORPHENESIN (UNII: I670DAL4SZ) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE SODIUM (UNII: MP1J8420LU) COCONUT ALCOHOL (UNII: 13F4MW8Y9K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WHEAT GERM OIL (UNII: 14C97E680P) CORN OIL (UNII: 8470G57WFM) DIMETHICONE (UNII: 92RU3N3Y1O) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) SUGARCANE (UNII: 81H2R5AOH3) ACER SACCHARUM SAP (UNII: 75UOH57984) BILBERRY (UNII: 9P2U39H18W) SUNFLOWER OIL (UNII: 3W1JG795YI) SOY PROTEIN (UNII: R44IWB3RN5) SOYBEAN OIL (UNII: 241ATL177A) MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) ALMOND (UNII: 3Z252A2K9G) LEMON (UNII: 24RS0A988O) LAVENDER OIL (UNII: ZBP1YXW0H8) ROSMARINUS OFFICINALIS FLOWERING TOP OIL (UNII: OXN0D3N28L) CUPRESSUS SEMPERVIRENS LEAF OIL (UNII: M7QUY89S4O) THYME OIL (UNII: 2UK410MY6B) ORANGE (UNII: 5EVU04N5QU) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) SODIUM BENZOATE (UNII: OJ245FE5EU) BETASIZOFIRAN (UNII: 2X51AD1X3T) METHYLPROPANEDIOL (UNII: N8F53B3R4R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51191-3016-1 50 g in 1 TUBE; Type 0: Not a Combination Product 04/15/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/15/2013 Labeler - MULTALER ET CIE S.A.S. (509613345) Registrant - MULTALER ET CIE S.A.S. (509613345) Establishment Name Address ID/FEI Business Operations MULTALER ET CIE S.A.S. 509613345 manufacture(51191-3016) , label(51191-3016)