Label: CARMEX DAILY CARE MOISTURIZING LIP BALM SPF 15 ASSORTED- homosalate, octocrylene, octisalate, avobenzone kit
- NDC Code(s): 10210-0082-1, 10210-0083-1, 10210-0084-1, 10210-0085-1
- Packager: Carma Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
• apply generously and evenly 15 minutes before sun exposure
• children under 6 months: Ask a doctor
• reapply: as needed or after towel drying, swimming or sweatingSun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.• wear long-sleeved shirts, pants, hats and sunglasses
-
Inactve ingredients
Wintergreen:petrolatum, lanolin, euphorbia cerifera (candelilla) wax, ozokerite, beeswax, cetyl esters, paraffin, theobroma cacao (cocoa) seed butter, flavor, isopropyl palmitate, limnanthes alba (meadowfoam) seed oil, stevia rebaudiana extract
Fresh Cherry, Strawberry:
petrolatum, lanolin, euphorbia cerifera (candelilla) wax, ozokerite, flavor, beeswax, cetyl esters, paraffin, theobroma cacao (cocoa) seed butter, isopropyl palmitate, limnanthes alba (meadowfoam) seed oil, stevia rebaudiana extract - Package Labeling:
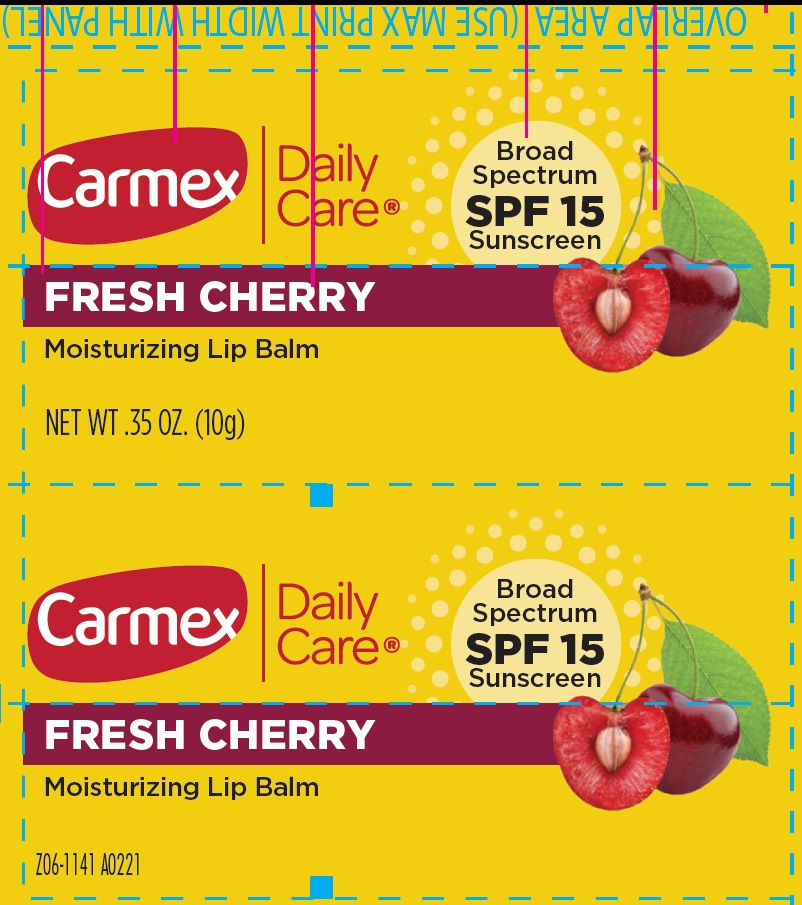
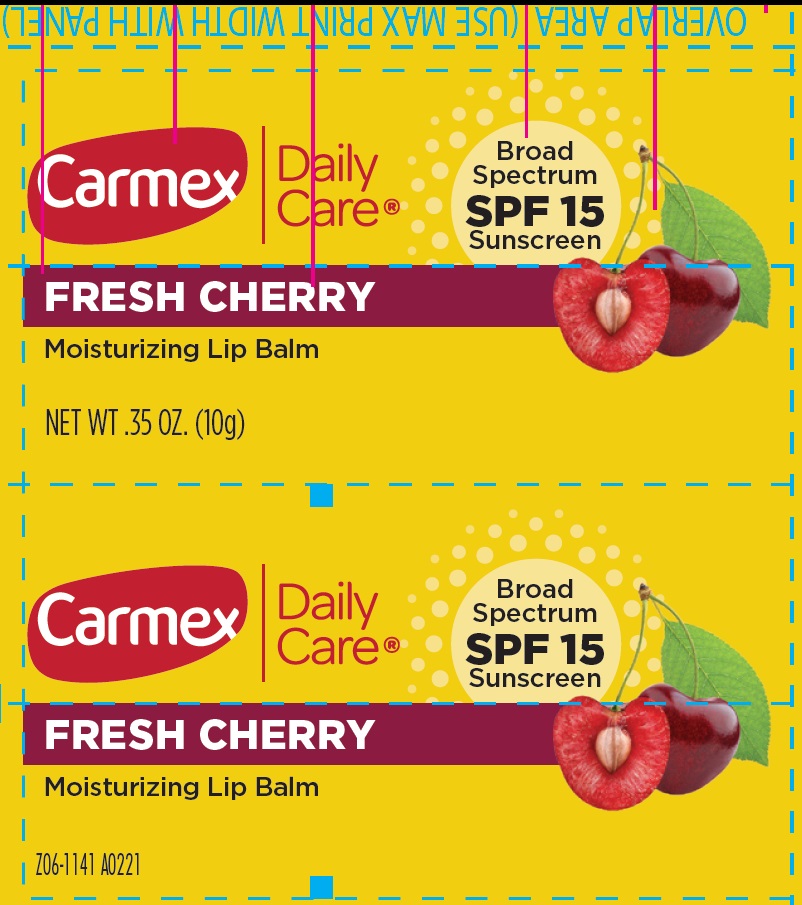
- Package Labeling:FRESH CHERRY
- Package Labeling:STRAWBERRY
- Package Labeling:WINTERGREEN
-
INGREDIENTS AND APPEARANCE
CARMEX DAILY CARE MOISTURIZING LIP BALM SPF 15 ASSORTED
homosalate, octocrylene, octisalate, avobenzone kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10210-0082 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0082-1 1 in 1 TRAY 01/01/2023 1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 10 g Part 2 2 TUBE 20 g Part 3 1 TUBE 10 g Part 1 of 3 CARMEX DAILY CARE MOISTURIZING LIP BALM SPF 15 FRESH CHERRY
homosalate, octocrylene, octisalate, avobenzone salveProduct Information Item Code (Source) NDC:10210-0083 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 70 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 53 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 33 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 24 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) LANOLIN (UNII: 7EV65EAW6H) CANDELILLA WAX (UNII: WL0328HX19) YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ESTERS WAX (UNII: D072FFP9GU) PARAFFIN (UNII: I9O0E3H2ZE) COCOA BUTTER (UNII: 512OYT1CRR) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0083-1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2023 Part 2 of 3 CARMEX DAILY CARE MOISTURIZING LIP BALM SPF 15 STRAWBERRY
homosalate, octocrylene, octisalate, avobenzone salveProduct Information Item Code (Source) NDC:10210-0084 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 70 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 53 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 33 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 24 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) LANOLIN (UNII: 7EV65EAW6H) CANDELILLA WAX (UNII: WL0328HX19) YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ESTERS WAX (UNII: D072FFP9GU) PARAFFIN (UNII: I9O0E3H2ZE) COCOA BUTTER (UNII: 512OYT1CRR) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0084-1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2023 Part 3 of 3 CARMEX DAILY CARE MOISTURIZING LIP BALM SPF 15 WINTERGREEN
homosalate, octocrylene, octisalate, avobenzone salveProduct Information Item Code (Source) NDC:10210-0085 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 70 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 53 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 33 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 24 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) LANOLIN (UNII: 7EV65EAW6H) CANDELILLA WAX (UNII: WL0328HX19) YELLOW WAX (UNII: 2ZA36H0S2V) CETYL ESTERS WAX (UNII: D072FFP9GU) PARAFFIN (UNII: I9O0E3H2ZE) COCOA BUTTER (UNII: 512OYT1CRR) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10210-0085-1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2023 Labeler - Carma Laboratories, Inc. (006090153)