Label: WAL-SOM- diphenhydramine hydrochloride tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 0363-0223-01, 0363-0223-02 - Packager: Walgreen
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 22, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Do not use
- For children under 12 years of age
- With any other product containing Diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- Directions
- Other information
- Inactive ingredients
- Question or comments?
-
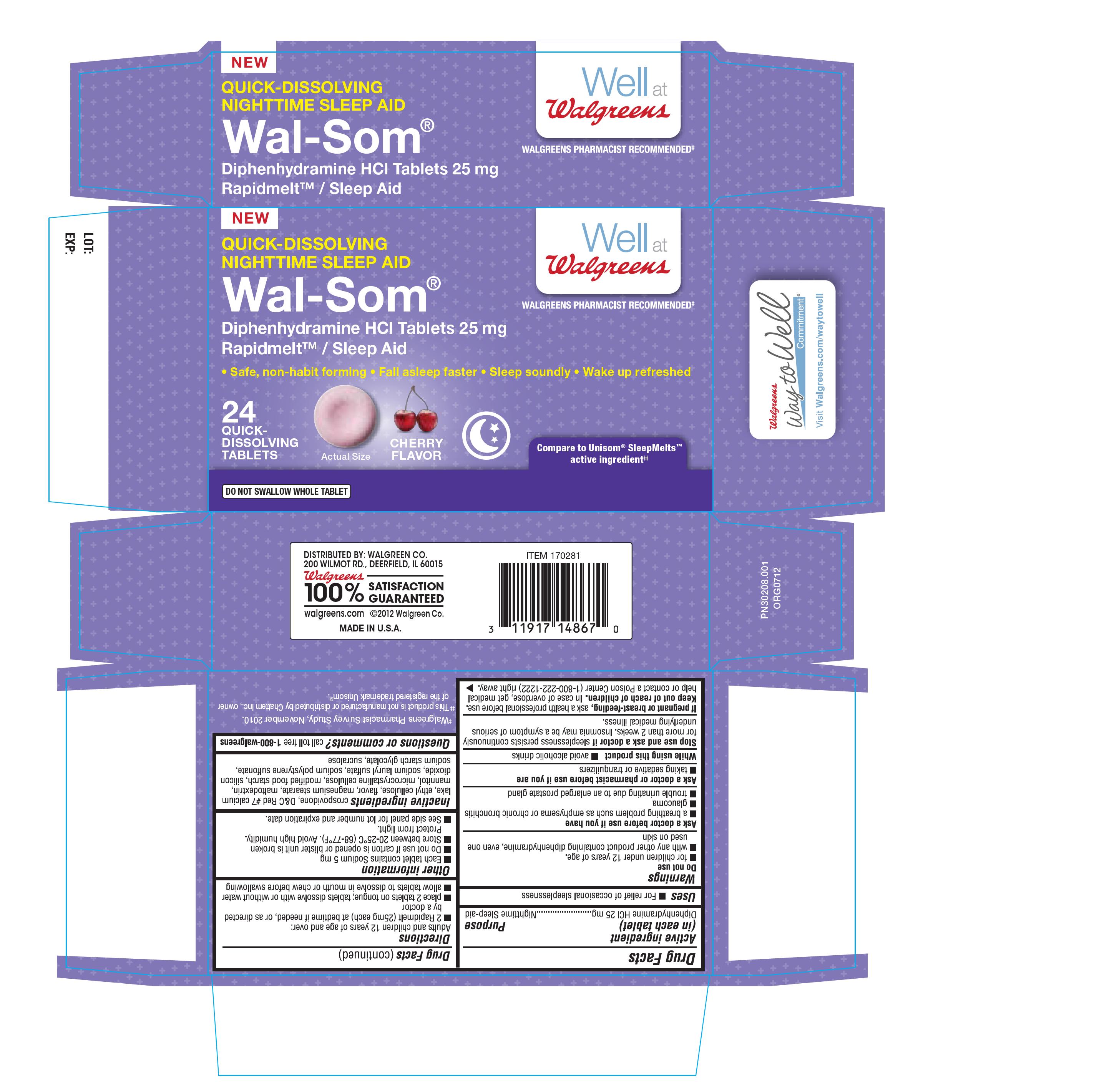
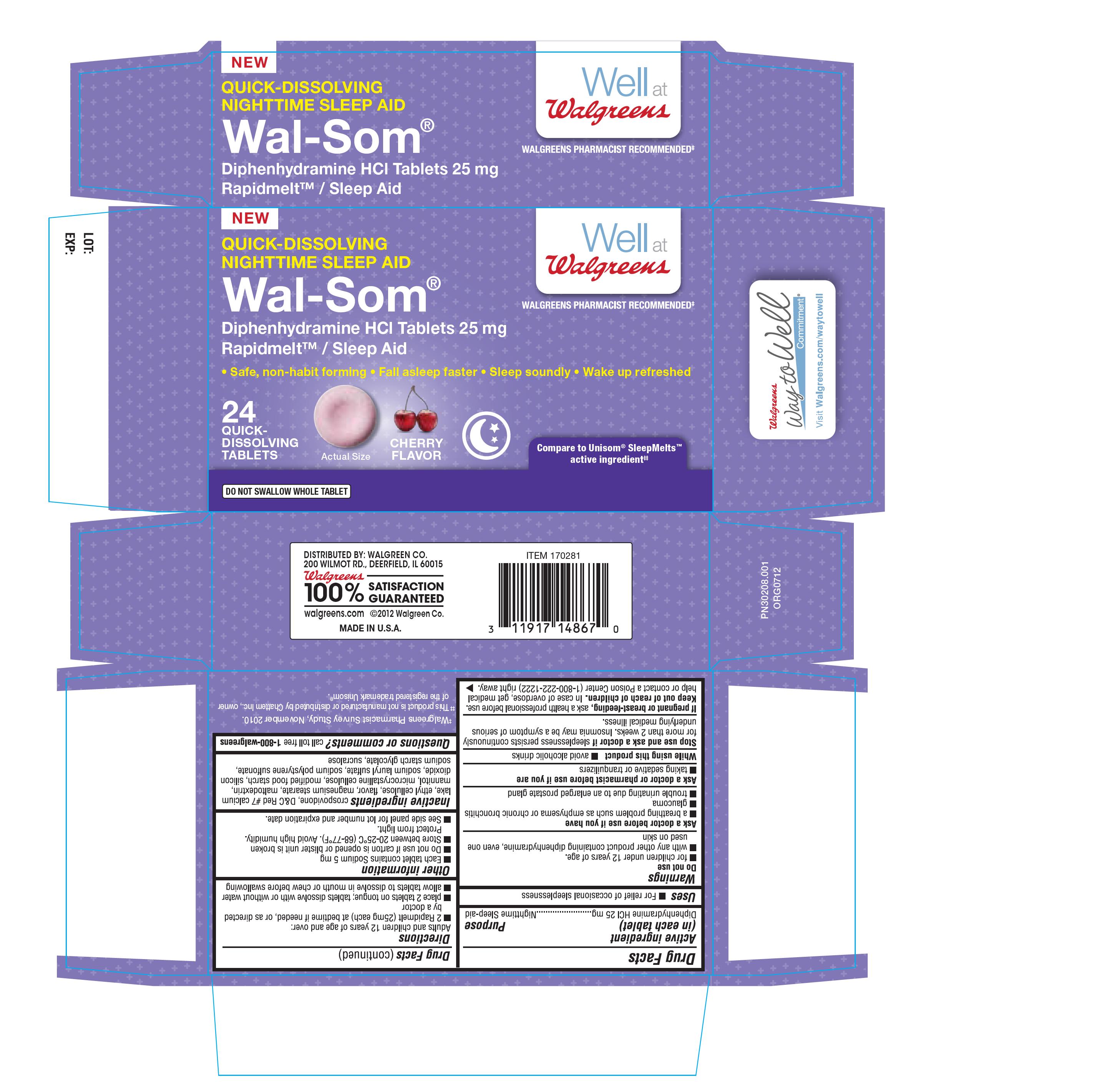
Principal display panel
NEW
QUICK DISSOLVING
NIGHTTIME SLEEP AID
WELL AT WALGREENS
WALGREENS PHARMACIST RECOMMENDED
WAL-SOM
DIPHENHYDRAMINE HCL TABLETS 25MG
RAPIDMELT/ SLEEP AID
SAFE, NON-HABIT FORMING, FALL ASLEEP FASTER, SLEEP SOUNDLY, WAKE UP REFRESHED
24 QUICK DISSOLVING TABLETS
COMPARE TO UNISOM SLEEPMELTS ACTIVE INGREDIENT
DO NOT SWALLOW WHOLE TABLET
-
INGREDIENTS AND APPEARANCE
WAL-SOM
diphenhydramine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-0223 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 68401960MK) D&C RED NO. 7 (UNII: ECW0LZ41X8) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MANNITOL (UNII: 3OWL53L36A) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SUCRALOSE (UNII: 96K6UQ3ZD4) MODIFIED CORN STARCH (1-OCTENYL SUCCINIC ANHYDRIDE) (UNII: 461P5CJN6T) Product Characteristics Color pink (pink color) Score no score Shape ROUND (Round lozenge shaped) Size 14mm Flavor CHERRY (Cherry flavor) Imprint Code CP223 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-0223-02 4 in 1 CARTON 1 NDC:0363-0223-01 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part338 08/22/2012 Labeler - Walgreen (008965063) Registrant - Capricorn Pharma Inc. (041704524) Establishment Name Address ID/FEI Business Operations Capricorn Pharma Inc. 041704524 manufacture(0363-0223) , analysis(0363-0223) , pack(0363-0223) , label(0363-0223)